Sleep: Difference between revisions
Groupuscule (talk | contribs) small copyedits and clarifications; give dream and industrialization each a sentence in the lead; move text rejecting energy hypothesis for sleep purpose out of lead. |
Groupuscule (talk | contribs) restructure top of article for clarity (and to create a framework which will accommodate more content); consolidate two listings of nrem/rem sleep stages; add some facts from recent literature review and other sources |
||
| Line 3: | Line 3: | ||
{{Redirect10|Waking up|Asleep|Slept|other uses|[[Waking Up (disambiguation)]], [[Asleep (disambiguation)]], and [[SLEPT analysis]]}} |
{{Redirect10|Waking up|Asleep|Slept|other uses|[[Waking Up (disambiguation)]], [[Asleep (disambiguation)]], and [[SLEPT analysis]]}} |
||
[[File:Sleeping-girl.jpg|thumb|Sleeping is associated with a state of [[muscle relaxation]] and limited perception of environmental stimuli.]] |
[[File:Sleeping-girl.jpg|thumb|Sleeping is associated with a state of [[muscle relaxation]] and limited perception of environmental stimuli.]] |
||
'''Sleep''' is a naturally recurring state of animals characterized by altered [[consciousness]], relatively inhibited sensory activity, and inhibition of nearly all [[voluntary muscle]]s.<ref>''Macmillan Dictionary for Students'' Macmillan, Pan Ltd. (1981), p. 936. Retrieved 1 October 2009.</ref> It is distinguished from [[wakefulness]] by a decreased ability to react to [[stimulus (physiology)|stimuli]], but is more easily reversed than the state of [[hibernation]] or of being [[coma]]tose. |
'''Sleep''' is a naturally recurring state of animals characterized by altered [[consciousness]], relatively inhibited sensory activity, and inhibition of nearly all [[voluntary muscle]]s.<ref>''Macmillan Dictionary for Students'' Macmillan, Pan Ltd. (1981), p. 936. Retrieved 1 October 2009.</ref> It is distinguished from [[wakefulness]] by a decreased ability to react to [[stimulus (physiology)|stimuli]], but is more easily reversed than the state of [[hibernation]] or of being [[coma]]tose. Mammalian sleep occurs in repeating periods, in which the body alternates between two highly distinct modes known as [[non-REM sleep|non-REM]] and [[rapid eye movement sleep|REM]] sleep. REM stands for “rapid eye movement” but involves many other aspects including virtual paralysis of the body. |
||
During sleep, most systems in an animal are in a heightened [[anabolic]] state, accentuating the growth and rejuvenation of the immune, nervous, skeletal, and muscular systems. [[Sleep (non-human)|Sleep in non-human animals]] is observed in mammals, birds, [[reptiles]], amphibians, and fish, and in some form in insects and even in simpler animals such as nematodes. The internal [[circadian clock]] tends to promote sleep during a regular time of day or night. However, sleep patterns vary widely among animals and within the human species. Industrialization and artificial light have substantially altered human sleep habits. |
During sleep, most systems in an animal are in a heightened [[anabolic]] state, accentuating the growth and rejuvenation of the immune, nervous, skeletal, and muscular systems. [[Sleep (non-human)|Sleep in non-human animals]] is observed in mammals, birds, [[reptiles]], amphibians, and fish, and in some form in insects and even in simpler animals such as nematodes. The internal [[circadian clock]] tends to promote sleep during a regular time of day or night. However, sleep patterns vary widely among animals and within the human species. Industrialization and artificial light have substantially altered human sleep habits. |
||
| Line 11: | Line 11: | ||
{{TOC limit|limit=3}} |
{{TOC limit|limit=3}} |
||
==Physiology== |
== Physiology == |
||
[[File:Sleep Hypnogram.svg|thumb|Hypnogram showing sleep cycles from midnight to 6.30 am, with deep sleep early on. There is more REM (marked red) before waking. (Current hypnograms reflect the recent decision to combine NREM stages 3 & 4 into a single stage 3.)|alt=Hypnogram showing sleep cycles from midnight to morning.]] |
[[File:Sleep Hypnogram.svg|thumb|Hypnogram showing sleep cycles from midnight to 6.30 am, with deep sleep early on. There is more REM (marked red) before waking. (Current hypnograms reflect the recent decision to combine NREM stages 3 & 4 into a single stage 3.)|alt=Hypnogram showing sleep cycles from midnight to morning.]] |
||
In mammals and birds, sleep is divided into two broad types: [[rapid eye movement sleep|rapid eye movement]] (REM sleep) and [[non-rapid eye movement sleep|non-rapid eye movement]] (NREM or non-REM sleep). Each type has a distinct set of physiological and neurological features associated with it. REM sleep is associated with dreaming, desynchronized and faster brain waves, loss of muscle tone, and suspension of homeostasis.<ref name="National">{{cite web|url=http://www.ninds.nih.gov/disorders/brain_basics/understanding_sleep.htm#dreaming|title=Brain Basics: Understanding Sleep|work=nih.gov}}</ref> |
In mammals and birds, sleep is divided into two broad types: [[rapid eye movement sleep|rapid eye movement]] (REM sleep) and [[non-rapid eye movement sleep|non-rapid eye movement]] (NREM or non-REM sleep). Each type has a distinct set of physiological and neurological features associated with it. REM sleep is associated with dreaming, desynchronized and faster brain waves, loss of muscle tone, and suspension of homeostasis.<ref name="National">{{cite web|url=http://www.ninds.nih.gov/disorders/brain_basics/understanding_sleep.htm#dreaming|title=Brain Basics: Understanding Sleep|work=nih.gov}}</ref> REM and non-REM sleep are so different that physiologists classify them as distinct behavioral states. In this view, REM, non-REM, and waking represent the three major modes of consciousness, neural activity, and physiological regulation.<ref name=HobsonEtAl2000>J. Alan Hobson, Edward F. Pace-Scott, & Robert Stickgold (2000), “Dreaming and the brain: Toward a cognitive neuroscience of conscious states”, ''Behavioral and Brain Sciences'' 23.</ref> According to the Hobson & McCarley [[activation-synthesis hypothesis]], proposed in 1975–1977, the alternation between REM and non-REM can be explained in terms of cycling, reciprocally influential neurotransmitter systems.<ref name=HobsonMcCarley1977>J. Alan Hobson & Robert W. McCarley, “The Brain as a Dream-State Generator: An Activation-Synthesis Hypothesis of the Dream Process”, ''American Journal of Psychiatry'' 134.12, December 1977.</ref> |
||
Especially during non-REM sleep, the brain uses significantly less energy during sleep than it does in waking. In areas with reduced activity, the brain restores its supply of [[adenosine triphosphate]] (ATP), the molecule used for short-term storage and transport of energy.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, p. 1118–1119. “Compared with wakefulness, sleep reduces brain energy demands, as suggested by the 44% reduction in the cerebral metabolic rate (CMR) of glucose (791) and a 25% reduction in the CMR of O<sub>2</sub> (774) during sleep.”</ref> |
|||
=== Stages === |
|||
{{Further|Neural oscillation}} |
|||
Key physiological indicators in sleep include EEG of [[neural oscillations|brain waves]], [[electrooculography]] (EOG) of eye movements, and [[electromyography]] (EMG) of [[skeletal muscle]] activity. Simultaneous collection of these measurements is called [[polysomnography]] and can be performed in a specialized sleep laboratory.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, p. 1087.</ref> |
|||
* NREM stage 1: This is a stage of sleep that usually occurs between sleep and wakefulness, and sometimes occurs between periods of deeper sleep and periods of REM. The muscles are active, and the eyes roll slowly, opening and closing moderately. |
|||
* NREM stage 2: In this stage, [[Theta rhythm|theta activity]] is observed and sleepers become gradually harder to awaken; the [[alpha wave]]s of the previous stage are interrupted by abrupt activity called [[sleep spindle]]s and [[K-complex]]es.<ref name=Schacter>Schacter, Daniel L.; Gilbert, Daniel T. and Wegner, Daniel M. (2009) ''Psychology'', Worth Publishers, ISBN 1429206152</ref> |
|||
* NREM stage 3: Formerly divided into stages 3 and 4, this stage is called [[slow-wave sleep]] (SWS). SWS is initiated in the [[preoptic area]] and consists of [[delta activity]], high amplitude waves at less than 3.5 Hz. The sleeper is less responsive to the environment; many environmental stimuli no longer produce any reactions. |
|||
* REM: The sleeper now enters rapid eye movement (REM) where most muscles are paralyzed. REM sleep is turned on by [[acetylcholine]] secretion and is inhibited by [[neurons]] that secrete [[serotonin]]. This level is also referred to as ''paradoxical sleep'' because the sleeper, although exhibiting EEG waves similar to a waking state, is harder to arouse than at any other sleep stage. Vital signs indicate arousal and oxygen consumption by the brain is higher than when the sleeper is awake.<ref name="Saladin 2012 537">{{cite book|last=Saladin|first=Kenneth S.|title=Anatomy and Physiology: The Unity of Form and Function, 6th Edition|year=2012|publisher=McGraw-Hill|isbn=978-0-07-337825-1|pages=537}}</ref> An adult reaches REM approximately every 90 minutes, with the latter half of sleep being more dominated by this stage. REM sleep occurs as a person returns to stage 1 from a deep sleep.<ref name ="National"/> The function of REM sleep is uncertain but a lack of it impairs the ability to learn complex tasks. One approach to understanding the role of sleep is to study the deprivation of it.<ref>{{cite book | author = Carlson NR, Miller HL, Heth DS, Donahoe JW, Martin GN | title = Psychology The Science of Behavior, Books a La Carte Edition. | date = 2010 | publisher = Pearson College Div | isbn = 0205762239 }}</ref> During periods of REM the EEG pattern returns to high-frequency waves that look similar to the waves produced while the person is awake.<ref name=Schacter/> |
|||
== Stages == |
|||
[[File:Sleep Stage N3.png|thumb|30 seconds of deep (stage N3) sleep.]] |
[[File:Sleep Stage N3.png|thumb|30 seconds of deep (stage N3) sleep.]] |
||
[[File:Sleep Stage REM.png|thumb|A screenshot of a PSG of a person in REM sleep. Eye movements highlighted by red box.]] |
[[File:Sleep Stage REM.png|thumb|A screenshot of a PSG of a person in REM sleep. Eye movements highlighted by red box.]] |
||
Sleep proceeds in cycles of REM and NREM, usually four or five of them per night, the order normally being N1 → N2 → N3 → N2 → REM. There is a greater amount of deep sleep (stage N3) earlier in the night, while the proportion of REM sleep increases in the two cycles just before natural awakening. |
|||
Human sleep occurs in periods of approximately 90 minutes, which include an increasing proportion of paradoxical (REM) sleep as they repeat. This rhythm is called the ''ultradian sleep cycle''.<ref>Parmeggiani (2011), ''Systemic Homeostasis and Poikilostasis in Sleep'', p. 9–11.</ref> Sleep proceeds in cycles of REM and NREM, usually four or five of them per night. The [[American Academy of Sleep Medicine]] (AASM) divides NREM into three stages: N1, N2, and N3, the last of which is also called delta sleep or slow-wave sleep.<ref>{{cite journal | author = Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C | title = The visual scoring of sleep in adults | journal = Journal of Clinical Sleep Medicine | volume = 3 | issue = 2 | pages = 121–31 | date = March 2007 | pmid = 17557422 | url = http://www.aasmnet.org/jcsm/Articles/030203.pdf }}</ref> The whole period normally proceeds in the order: N1 → N2 → N3 → N2 → REM. In other animals the subdivision between phases of non-REM sleep is not typically used, although animal non-REM sleep can be described as ligher or deeper.<ref name=Brown2012Page1108>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, pp. 1108–1109.</ref> There is a greater amount of deep sleep (stage N3) earlier in the night, while the proportion of REM sleep increases in the two cycles just before natural awakening. |
|||
The stages of sleep were first described in 1937 by [[Alfred Lee Loomis]] and his coworkers, who separated the different [[electroencephalography]] (EEG) features of sleep into five levels (A to E), which represented the spectrum from wakefulness to deep sleep.<ref>{{cite journal | author = Loomis AL, Harvey EN, Hobart GA | title = III Cerebral states during sleep, as studied by human brain potentials | journal = J Exp Psychol. | year = 1937 | volume = 21 | issue = 2 | pages = 127–44 | doi = 10.1037/h0057431 }}</ref> In 1953, REM sleep was discovered as distinct, and thus [[William Dement]] and [[Nathaniel Kleitman]] reclassified sleep into four NREM stages and REM.<ref>{{cite journal | author = Dement W, Kleitman N | title = Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming | journal = Electroencephalogr Clin Neurophysiol | volume = 9 | issue = 4 | pages = 673–90 | year = 1957 | pmid = 13480240 | doi = 10.1016/0013-4694(57)90088-3 }}</ref> The staging criteria were standardized in 1968 by [[Allan Rechtschaffen]] and Anthony Kales in the "R&K sleep scoring manual."<ref>{{cite book | url=http://commons.wikimedia.org/wiki/File:Rechschaffen-Kales---Manual.pdf | author=Rechtschaffen A, Kales A, editors. | title=A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. | publisher=Washington: Public Health Service, US Government Printing Office | year=1968 | format = PDF}}</ref> |
|||
Each stage may have a distinct physiological function and this can result in sleep that exhibits [[unconsciousness|loss of consciousness]] but does not fulfill its physiological functions (i.e., one may still feel tired after apparently sufficient sleep). |
|||
In the R&K standard, NREM sleep was divided into four stages, with slow-wave sleep comprising stages 3 and 4. In stage 3, delta waves made up less than 50% of the total wave patterns, while they made up more than 50% in stage 4. Furthermore, REM sleep was sometimes referred to as stage 5. |
|||
=== Non-REM === |
|||
In 2004, the AASM commissioned the AASM Visual Scoring Task Force to review the R&K scoring system. The review resulted in several changes, the most significant being the combination of stages 3 and 4 into Stage N3. The revised scoring was published in 2007 as ''The AASM Manual for the Scoring of Sleep and Associated Events''.<ref>{{cite book |last=Iber |first=C |author2=Ancoli-Israel, S |author3=Chesson, A |author4= Quan, SF for the American Academy of Sleep Medicine |title=The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications |location=Westchester |publisher=American Academy of Sleep Medicine |year=2007}}</ref> Arousals and respiratory, cardiac, and movement events were also added.<ref>{{cite web |url=http://web.mst.edu/~psyworld/general/sleepstages/sleepstages.pdf |title= Stages of Sleep |accessdate=15 June 2008 |author=Psychology World |year=1998 |format=PDF |quote=(includes illustrations of "sleep spindles" and "K-complexes")}}</ref><ref>{{cite journal | author = Schulz H | title = Rethinking sleep analysis | journal = Journal of Clinical Sleep Medicine | volume = 4 | issue = 2 | pages = 99–103 | date = April 2008 | pmid = 18468306 | pmc = 2335403 }}</ref> |
|||
{{Main|Non-REM sleep}} |
|||
As an awake organism falls asleep, the activity of its body slows down. Body temperature, heart rate, breathing rate, and energy use all decrease. [[Brain waves]] get slower and bigger. The excitatory neurotransmitter [[acetylcholine]] becomes less available in the brain.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, pp. 1100–1102.</ref> The organism will maneuver, as best it can, to create a thermally friendly environment—for example, by curling up into a ball if it's cold out. Reflexes remain fairly active. These characteristics apply to some degree during all non-REM sleep, which constitutes ~80% of all sleep in humans.<ref>Parmeggiani (2011), ''Systemic Homeostasis and Poikilostasis in Sleep'', ''passim''.</ref> |
|||
==== NREM 1 ==== |
|||
Sleep stages and other characteristics of sleep are commonly assessed by [[polysomnography]] in a specialized sleep laboratory. Measurements taken include EEG of [[neural oscillations|brain waves]], [[electrooculography]] (EOG) of eye movements, and [[electromyography]] (EMG) of [[skeletal muscle]] activity. In humans, the average length of the first sleep cycle is approximately 90 minutes and 100 to 120 minutes from the second to the fourth cycle, which is usually the last one.<ref>{{cite book|publisher=Springer|author=Guilleminault, C. and Kreutzer, M.L. |editor=Michael Billiard|accessdate=7 April 2012|url=http://books.google.com/books?id=IorPrIY6dOYC&lpg=PA|page=5|chapter=Chapter 1 – Normal Sleep|quote=The average length of the first sleep cycle is approximately 90 minutes and 100 to 120 minutes from the second to the fourth cycle, which is usually the last one.|title=Sleep: Physiology, Investigations, and Medicine (Google eBook)|isbn=978-0-306-47406-4|date=30 September 2003}}</ref> Each stage may have a distinct physiological function and this can result in sleep that exhibits [[unconsciousness|loss of consciousness]] but does not fulfill its physiological functions (i.e., one may still feel tired after apparently sufficient sleep). |
|||
NREM stage 1 (N1): This is a stage of sleep that usually occurs between sleep and wakefulness, and sometimes occurs between periods of deeper sleep and periods of REM. The muscles are active, and the eyes roll slowly, opening and closing moderately. The brain transitions from [[alpha waves]] having a frequency of 8–13 [[Hertz|Hz]] (common in the awake state) to [[theta wave]]s having a frequency of 4–7 Hz. This stage is sometimes referred to as somnolence or drowsy sleep. Sudden twitches and [[hypnic jerk]]s, also known as positive [[myoclonus]], may be associated with the onset of sleep during N1. Some people may also experience [[hypnagogic hallucination]]s during this stage. During N1, the organism loses some [[muscle tone]] and most conscious awareness of the external environment. |
|||
Scientific studies on sleep have shown that sleep stage at awakening is an important factor in amplifying [[sleep inertia]].{{Citation needed|date=March 2014}} [[Alarm clock]]s involving ''sleep stage monitoring'' appeared on the market in 2005.<ref>{{cite news |url=http://www.reuters.com/article/technologyNews/idUSL0878172320070829 |title=Bio-alarm clocks set for perfect wake-up |accessdate=9 June 2008 |author=Fenton, Reuven |work= Reuters |date=29 August 2007}}</ref> Using sensing technologies such as [[EEG]] electrodes or [[accelerometers]], these alarm clocks are supposed to wake people only from light sleep. |
|||
===NREM |
==== NREM 2 ==== |
||
{{Main|Non-rapid eye movement sleep}} |
|||
NREM stage 2 (N2): In this stage, [[Theta rhythm|theta activity]] is observed and sleepers become gradually harder to awaken; the [[alpha wave]]s of the previous stage are interrupted by abrupt activity called [[sleep spindle]]s (or thalamocortical spindles) and [[K-complex]]es.<ref name=Schacter>Schacter, Daniel L.; Gilbert, Daniel T. and Wegner, Daniel M. (2009) ''Psychology'', Worth Publishers, ISBN 1429206152</ref> Sleep spindles range from 11 to 16 Hz (most commonly 12–14 Hz) and [[K-complex]]es. During this stage, muscular activity as measured by EMG decreases, and conscious awareness of the external environment disappears. This stage occupies 45–55% of total sleep in adults. |
|||
According to 2007 AASM standards, NREM consists of three stages. There is relatively little dreaming in NREM. |
|||
==== NREM 3 ==== |
|||
'''Stage N1''' refers to the transition of the brain from [[alpha waves]] having a frequency of 8–13 [[Hertz|Hz]] (common in the awake state) to [[theta wave]]s having a frequency of 4–7 Hz. This stage is sometimes referred to as somnolence or drowsy sleep. Sudden twitches and [[hypnic jerk]]s, also known as positive [[myoclonus]], may be associated with the onset of sleep during N1. Some people may also experience [[hypnagogic hallucination]]s during this stage. During N1, the subject loses some [[muscle tone]] and most conscious awareness of the external environment. |
|||
{{Main|Slow-wave sleep}} |
|||
NREM stage 3: Formerly divided into stages 3 and 4, this stage is called [[slow-wave sleep]] (SWS) or deep sleep. SWS is initiated in the [[preoptic area]] and consists of [[delta activity]], high amplitude waves at less than 3.5 Hz. The sleeper is less responsive to the environment; many environmental stimuli no longer produce any reactions. |
|||
'''Stage N2''' is characterized by [[sleep spindle]]s ranging from 11 to 16 Hz (most commonly 12–14 Hz) and [[K-complex]]es. During this stage, muscular activity as measured by EMG decreases, and conscious awareness of the external environment disappears. This stage occupies 45–55% of total sleep in adults. |
|||
This stage is characterized by the presence of a minimum of 20% [[delta wave]]s ranging from 0.5–2 Hz and having a peak-to-peak amplitude >75 μV. (EEG standards define delta waves to be from 0 to 4 Hz, but sleep standards in both the original R&K, as well as the new 2007 AASM guidelines have a range of 0.5–2 Hz.) This is the stage in which parasomnias such as [[night terror]]s, [[nocturnal enuresis]], [[sleepwalking]], and [[somniloquy]] occur. Many illustrations and descriptions still show a stage N3 with 20–50% delta waves and a stage N4 with greater than 50% delta waves; these have been combined as stage N3. |
|||
===REM |
=== REM === |
||
{{Main|Rapid eye movement sleep}} |
{{Main|Rapid eye movement sleep}} |
||
REM: The sleeper now enters rapid eye movement (REM) where most muscles are paralyzed. REM sleep is turned on by [[acetylcholine]] secretion and is inhibited by [[neurons]] that secrete [[serotonin]]. This level is also referred to as ''paradoxical sleep'' because the sleeper, although exhibiting EEG waves similar to a waking state, is harder to arouse than at any other sleep stage. Vital signs indicate arousal and oxygen consumption by the brain is higher than when the sleeper is awake.<ref name="Saladin 2012 537">{{cite book|last=Saladin|first=Kenneth S.|title=Anatomy and Physiology: The Unity of Form and Function, 6th Edition|year=2012|publisher=McGraw-Hill|isbn=978-0-07-337825-1|pages=537}}</ref> An adult reaches REM approximately every 90 minutes, with the latter half of sleep being more dominated by this stage. REM sleep occurs as a person returns to stage 1 from a deep sleep.<ref name ="National"/> The function of REM sleep is uncertain but a lack of it impairs the ability to learn complex tasks. One approach to understanding the role of sleep is to study the deprivation of it.<ref>{{cite book | author = Carlson NR, Miller HL, Heth DS, Donahoe JW, Martin GN | title = Psychology The Science of Behavior, Books a La Carte Edition. | date = 2010 | publisher = Pearson College Div | isbn = 0205762239 }}</ref> During periods of REM the EEG pattern returns to high-frequency waves that look similar to the waves produced while the person is awake.<ref name=Schacter/> |
|||
Rapid eye movement sleep, or REM sleep (also known as paradoxical sleep),<ref name="Myers2003">{{cite book|author=David G. Myers|title=Psychology, Seventh Edition, in Modules (High School Version)|url=http://books.google.com/books?id=oYuBwPDsQZoC&pg=PA268|accessdate=22 August 2012|date=22 September 2003|publisher=Macmillan|isbn=978-0-7167-8595-8|pages=268–}}</ref> accounts for 20–25% of total sleep time in most human adults. The criteria for REM sleep include rapid eye movements as well as a rapid low-voltage EEG. During REM sleep, EEG patterns returns to higher frequency saw-tooth waves. Most memorable dreaming occurs in this stage. At least in mammals, a descending muscular [[atonia]] is seen. Such paralysis may be necessary to protect organisms from self-damage through physically acting out scenes from the often-vivid dreams that occur during this stage.{{Citation needed|date=June 2012}} |
Rapid eye movement sleep, or REM sleep (also known as paradoxical sleep),<ref name="Myers2003">{{cite book|author=David G. Myers|title=Psychology, Seventh Edition, in Modules (High School Version)|url=http://books.google.com/books?id=oYuBwPDsQZoC&pg=PA268|accessdate=22 August 2012|date=22 September 2003|publisher=Macmillan|isbn=978-0-7167-8595-8|pages=268–}}</ref> accounts for 20–25% of total sleep time in most human adults. The criteria for REM sleep include rapid eye movements as well as a rapid low-voltage EEG. During REM sleep, EEG patterns returns to higher frequency saw-tooth waves. Most memorable dreaming occurs in this stage. At least in mammals, a descending muscular [[atonia]] is seen. Such paralysis may be necessary to protect organisms from self-damage through physically acting out scenes from the often-vivid dreams that occur during this stage.{{Citation needed|date=June 2012}} |
||
A newborn baby spends almost 9 hours a day just in REM sleep. By the age of five or so, only slightly over two hours is spent in REM.<ref>{{cite web |last=Siegel |first=Jerome M |url=http://www.npi.ucla.edu/sleepresearch/encarta/Article.htm |title=Sleep |accessdate=25 January 2008 |year=1999 |work=Encarta Encyclopedia |publisher=Microsoft |archiveurl=http://web.archive.org/web/20071214003754/http://www.npi.ucla.edu/sleepresearch/encarta/Article.htm <!--Bot retrieved archive--> |archivedate=14 December 2007}}</ref> |
|||
===Timing=== |
|||
=== Awakening === |
|||
Scientific studies on sleep have shown that sleep stage at awakening is an important factor in amplifying [[sleep inertia]].{{Citation needed|date=March 2014}} [[Alarm clock]]s involving ''sleep stage monitoring'' appeared on the market in 2005.<ref>{{cite news |url=http://www.reuters.com/article/technologyNews/idUSL0878172320070829 |title=Bio-alarm clocks set for perfect wake-up |accessdate=9 June 2008 |author=Fenton, Reuven |work= Reuters |date=29 August 2007}}</ref> Using sensing technologies such as [[EEG]] electrodes or [[accelerometers]], these alarm clocks are supposed to wake people only from light sleep. |
|||
=== Historical development of stages model === |
|||
The stages of sleep were first described in 1937 by [[Alfred Lee Loomis]] and his coworkers, who separated the different [[electroencephalography]] (EEG) features of sleep into five levels (A to E), which represented the spectrum from wakefulness to deep sleep.<ref>{{cite journal | author = Loomis AL, Harvey EN, Hobart GA | title = III Cerebral states during sleep, as studied by human brain potentials | journal = J Exp Psychol. | year = 1937 | volume = 21 | issue = 2 | pages = 127–44 | doi = 10.1037/h0057431 }}</ref> In 1953, REM sleep was discovered as distinct, and thus [[William Dement]] and [[Nathaniel Kleitman]] reclassified sleep into four NREM stages and REM.<ref>{{cite journal | author = Dement W, Kleitman N | title = Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming | journal = Electroencephalogr Clin Neurophysiol | volume = 9 | issue = 4 | pages = 673–90 | year = 1957 | pmid = 13480240 | doi = 10.1016/0013-4694(57)90088-3 }}</ref> The staging criteria were standardized in 1968 by [[Allan Rechtschaffen]] and Anthony Kales in the "R&K sleep scoring manual."<ref>{{cite book | url=http://commons.wikimedia.org/wiki/File:Rechschaffen-Kales---Manual.pdf | author=Rechtschaffen A, Kales A, editors. | title=A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. | publisher=Washington: Public Health Service, US Government Printing Office | year=1968 | format = PDF}}</ref><ref name=Brown2012Page1108 /> |
|||
In the R&K standard, NREM sleep was divided into four stages, with slow-wave sleep comprising stages 3 and 4. In stage 3, delta waves made up less than 50% of the total wave patterns, while they made up more than 50% in stage 4. Furthermore, REM sleep was sometimes referred to as stage 5. In 2004, the AASM commissioned the AASM Visual Scoring Task Force to review the R&K scoring system. The review resulted in several changes, the most significant being the combination of stages 3 and 4 into Stage N3. The revised scoring was published in 2007 as ''The AASM Manual for the Scoring of Sleep and Associated Events''.<ref>{{cite book |last=Iber |first=C |author2=Ancoli-Israel, S |author3=Chesson, A |author4= Quan, SF for the American Academy of Sleep Medicine |title=The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications |location=Westchester |publisher=American Academy of Sleep Medicine |year=2007}}</ref> Arousals and respiratory, cardiac, and movement events were also added.<ref>{{cite web |url=http://web.mst.edu/~psyworld/general/sleepstages/sleepstages.pdf |title= Stages of Sleep |accessdate=15 June 2008 |author=Psychology World |year=1998 |format=PDF |quote=(includes illustrations of "sleep spindles" and "K-complexes")}}</ref><ref>{{cite journal | author = Schulz H | title = Rethinking sleep analysis | journal = Journal of Clinical Sleep Medicine | volume = 4 | issue = 2 | pages = 99–103 | date = April 2008 | pmid = 18468306 | pmc = 2335403 }}</ref> |
|||
== Circadian timing == |
|||
Sleep timing is controlled by the [[circadian clock]], sleep-wake [[homeostasis]], and in humans, within certain bounds, willed behavior. The circadian clock—an inner timekeeping, temperature-fluctuating, enzyme-controlling device—works in tandem with these other mechanisms. Circadian timing, known as process C, is cyclical, based on the time of day; sleep-wake homeostasis, or process S, operates on a more absolute scale. |
|||
=== Circadian clock === |
|||
{{Main|Circadian rhythm}} |
{{Main|Circadian rhythm}} |
||
[[image:Biological clock human.svg|thumb|The human "[[Circadian rhythm|biological clock]]"]] |
[[image:Biological clock human.svg|thumb|The human "[[Circadian rhythm|biological clock]]"]] |
||
Sleep timing is controlled by the [[circadian clock]], sleep-wake [[homeostasis]], and in humans, within certain bounds, willed behavior. The circadian clock—an inner timekeeping, temperature-fluctuating, enzyme-controlling device—works in tandem with [[adenosine]], a neurotransmitter that inhibits many of the bodily processes associated with wakefulness. Adenosine is created over the course of the day; high levels of adenosine lead to sleepiness.<ref>[http://thebrain.mcgill.ca/flash/a/a_11/a_11_m/a_11_m_cyc/a_11_m_cyc.html Molecules that build up and make you sleep]. thebrain.mcgill.ca</ref> |
|||
In [[diurnality|diurnal]] animals, sleepiness occurs as the circadian element causes the release of the hormone [[melatonin]] and a gradual decrease in core [[thermoregulation|body temperature]]. The timing is affected by one's [[chronotype]]. It is the circadian rhythm that determines the ideal timing of a correctly structured and restorative sleep episode.<ref>{{cite journal | author = Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ | title = Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day | journal = Am J Physiol | volume = 277 | issue = 4 | pages = R1152–R1163 | date = 1 October 1999 | pmid = 10516257 }}</ref> |
In [[diurnality|diurnal]] animals, sleepiness occurs as the circadian element causes the release of the hormone [[melatonin]] and a gradual decrease in core [[thermoregulation|body temperature]]. The timing is affected by one's [[chronotype]]. It is the circadian rhythm that determines the ideal timing of a correctly structured and restorative sleep episode.<ref>{{cite journal | author = Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ | title = Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day | journal = Am J Physiol | volume = 277 | issue = 4 | pages = R1152–R1163 | date = 1 October 1999 | pmid = 10516257 }}</ref> |
||
=== Distribution === |
|||
In [[polyphasic sleep]], an organism sleeps at multiple times during a 24-hour cycle. Monophasic sleep occurs all at once. |
|||
==== Naps==== |
|||
[[File:People sleeping in a train.jpg|thumb|People sleeping on a train at night]] |
|||
{{main|Nap}} |
|||
The [[siesta]] habit has recently been associated with a 37% reduction in coronary mortality, possibly due to reduced cardiovascular stress mediated by daytime sleep.<ref name=Naska/> Nevertheless, [[Epidemiology|epidemiological]] studies on the relations between cardiovascular health and siestas have led to conflicting conclusions, possibly because of poor control of [[moderator variable]]s, such as physical activity. It is possible that people who take siestas have different physical activity habits, e.g., waking earlier and scheduling more activity during the morning. Such differences in physical activity may [[Mediator variable|mediate]] different 24-hour profiles in cardiovascular function. Even if such effects of physical activity can be discounted for explaining the relationship between siestas and cardiovascular health, it is still unknown whether it is the daytime nap itself, a [[Supine position|supine]] posture, or the expectancy of a nap that is the most important factor. It was recently suggested that a short nap can reduce stress and blood pressure (BP), with the main changes in BP occurring between the time of lights off and the onset of stage 1.<ref name=Zaregarizi1/><ref name=Zaregarizi2/> |
|||
Dr. Zaregarizi and his team have concluded that the acute time of falling asleep was when beneficial cardiovascular changes take place. This study has indicated that a large decline in BP occurs during the daytime sleep-onset period only when sleep is expected. However, when subjects rest in a [[supine position]], the same reduction in BP is not observed. This BP reduction may be associated with the lower coronary mortality rates seen in Mediterranean and Latin American populations in which siestas are common. |
|||
Dr. Zaregarizi assessed cardiovascular function (BP, heart rate, and measurements of blood vessel dilation) while nine healthy volunteers, 34 years of age on average, spent an hour standing quietly, reclining at rest but not sleeping, or reclining to nap. All participants were restricted to 4 hours of sleep on the night prior to each of the sleep laboratory tests. During the three phases of daytime sleep, he noted significant reductions in BP and heart rate. By contrast, they did not observe changes in cardiovascular function while the participants were standing or reclining at rest. |
|||
These findings also show that the greatest decline in BP occurs between lights-off and onset of daytime sleep itself. |
|||
During this sleep period, which lasted 9.7 minutes on average, BP decreased, while blood vessel dilation increased by more than 9 percent. |
|||
“There is little change in blood pressure once a subject is actually asleep," Dr. Zaregarizi noted, and he found minor changes in blood vessel dilation during sleep.<ref name=Zaregarizi1/><ref name=Zaregarizi2/> |
|||
Sleep duration in long-term experienced meditators is lower than in non-meditators and general population norms, with no apparent decrements in vigilance.<ref>{{cite journal | author = Kaul P, Passafiume J, Sargent CR, O'Hara BF | title = Meditation acutely improves psychomotor vigilance, and may decrease sleep need | journal = Behav Brain Funct | volume = 6 | pages = 47 | year = 2010 | pmid = 20670413 | pmc = 2919439 | doi = 10.1186/1744-9081-6-47 | doi_brokendate = 2015-02-02 }}</ref> |
|||
== Sleep homeostasis, deprivation and optimization == |
|||
Generally speaking, the more an organism is awake, the more it feels a need to sleep. The balance between sleeping and waking is called [[homeostasis]]. Induced or perceived lack of sleep is commonly called [[sleep deprivation]]. |
|||
Sleep deprivation tends to cause slower brain waves in the frontal cortex, shortened attention span, higher anxiety, impaired memory, and a grouchy mood. Conversely, a well-rested organism tends to have improved memory and mood.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, pp. 1134–1138.</ref> |
|||
=== Duration === |
|||
Homeostatic sleep propensity (the need for sleep as a function of the amount of time elapsed since the last adequate sleep episode) must be balanced against the circadian element for satisfactory sleep.<ref name="Zisapel">{{cite journal | author = Zisapel N | title = Sleep and sleep disturbances: biological basis and clinical implications | journal = Cell Mol Life Sci | volume = 64 | issue = 10 | pages = 1174–86 | year = 2007 | pmid = 17364142 | doi = 10.1007/s00018-007-6529-9 }}</ref> Along with corresponding messages from the circadian clock, this tells the body it needs to sleep.<ref name="autogenerated1">{{cite web |url=http://www.helpguide.org/life/sleeping.htm |title=Understanding Sleep: Sleep Needs, Cycles, and Stages |accessdate=25 January 2008 |last=de Benedictis |first=Tina, PhD |coauthors=Heather Larson, Gina Kemp, MA, Suzanne Barston, Robert Segal, MA |year=2007 |publisher=Helpguide.org}}</ref> Sleep offset (awakening) is primarily determined by circadian rhythm. A person who regularly awakens at an early hour will generally not be able to sleep much later than his or her normal waking time, even if moderately sleep-deprived{{Citation needed|date=June 2013}}. |
Homeostatic sleep propensity (the need for sleep as a function of the amount of time elapsed since the last adequate sleep episode) must be balanced against the circadian element for satisfactory sleep.<ref name="Zisapel">{{cite journal | author = Zisapel N | title = Sleep and sleep disturbances: biological basis and clinical implications | journal = Cell Mol Life Sci | volume = 64 | issue = 10 | pages = 1174–86 | year = 2007 | pmid = 17364142 | doi = 10.1007/s00018-007-6529-9 }}</ref> Along with corresponding messages from the circadian clock, this tells the body it needs to sleep.<ref name="autogenerated1">{{cite web |url=http://www.helpguide.org/life/sleeping.htm |title=Understanding Sleep: Sleep Needs, Cycles, and Stages |accessdate=25 January 2008 |last=de Benedictis |first=Tina, PhD |coauthors=Heather Larson, Gina Kemp, MA, Suzanne Barston, Robert Segal, MA |year=2007 |publisher=Helpguide.org}}</ref> Sleep offset (awakening) is primarily determined by circadian rhythm. A person who regularly awakens at an early hour will generally not be able to sleep much later than his or her normal waking time, even if moderately sleep-deprived{{Citation needed|date=June 2013}}. |
||
| Line 69: | Line 105: | ||
Sleep duration is affected by the gene [[DEC2]]. People with a certain DEC2 mutation sleep two hours less than normal. The gene also affects the sleep patterns of mice, and likely does so for all mammals.<ref>{{cite web |url=http://www.medicinenet.com/script/main/art.asp?articlekey=104720|archiveurl=http://web.archive.org/web/20110714065601/http://www.medicinenet.com/script/main/art.asp?articlekey=104720|archivedate=14 July 2011|title=Gene Cuts Need for Sleep - Sleep Disorders Including, Sleep Apnea, Narcolepsy, Insomnia, Snoring and Nightmares on MedicineNet.com |accessdate=11 June 2010}}</ref><ref>{{cite journal | author = He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Rossner MJ, Nishino S, Fu YH | title = The transcriptional repressor DEC2 regulates sleep length in mammals | journal = Science | volume = 325 | issue = 5942 | pages = 866–70 | year = 2009 | pmid = 19679812 | pmc = 2884988 | doi = 10.1126/science.1174443 }}</ref> |
Sleep duration is affected by the gene [[DEC2]]. People with a certain DEC2 mutation sleep two hours less than normal. The gene also affects the sleep patterns of mice, and likely does so for all mammals.<ref>{{cite web |url=http://www.medicinenet.com/script/main/art.asp?articlekey=104720|archiveurl=http://web.archive.org/web/20110714065601/http://www.medicinenet.com/script/main/art.asp?articlekey=104720|archivedate=14 July 2011|title=Gene Cuts Need for Sleep - Sleep Disorders Including, Sleep Apnea, Narcolepsy, Insomnia, Snoring and Nightmares on MedicineNet.com |accessdate=11 June 2010}}</ref><ref>{{cite journal | author = He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Rossner MJ, Nishino S, Fu YH | title = The transcriptional repressor DEC2 regulates sleep length in mammals | journal = Science | volume = 325 | issue = 5942 | pages = 866–70 | year = 2009 | pmid = 19679812 | pmc = 2884988 | doi = 10.1126/science.1174443 }}</ref> |
||
=== |
=== Sleep debt === |
||
{{Main|Sleep debt}} |
|||
{{See also|Circadian rhythm#Biological markers}} |
|||
Sleep debt is the effect of not getting enough sleep; a large debt causes mental, emotional and physical fatigue.{{citation needed|date=October 2013}} |
|||
Sleep debt results in diminished abilities to perform high-level cognitive functions. Neurophysiological and functional [[imaging studies]] have demonstrated that frontal regions of the brain are particularly responsive to homeostatic sleep pressure.<ref>{{cite journal | author = Gottselig JM, Adam M, Rétey JV, Khatami R, Achermann P, Landolt HP | title = Random number generation during sleep deprivation: effects of caffeine on response maintenance and stereotypy | journal = Journal of Sleep Research | volume = 15 | issue = 1 | pages = 31–40 | date = March 2006 | pmid = 16490000 | doi = 10.1111/j.1365-2869.2006.00497.x }}</ref> |
|||
Scientists do not agree on how much sleep debt it is possible to accumulate; whether it is accumulated against an individual's average sleep or some other benchmark; nor on whether the prevalence of sleep debt among adults has changed appreciably in the [[developed country|industrialized world]] in recent decades. It is likely that children are sleeping less than previously in [[Western world|Western societies]].<ref>{{cite journal | author = Iglowstein I, Jenni OG, Molinari L, Largo RH | title = Sleep duration from infancy to adolescence: reference values and generational trends | journal = Pediatrics | volume = 111 | issue = 2 | pages = 302–7 | date = February 2003 | pmid = 12563055 | doi = 10.1542/peds.111.2.302 | quote = Thus, the shift in the evening bedtime across cohorts accounted for the substantial decrease in sleep duration in younger children between the 1970s and the 1990s... [A] more liberal parental attitude toward evening bedtime in the past decades is most likely responsible for the bedtime shift and for the decline of sleep duration... }}</ref> |
|||
One neurochemical indicator of sleep debt is [[adenosine]], a neurotransmitter that inhibits many of the bodily processes associated with wakefulness. Adenosine is an ingredient in [[adenosine triphosphate]] (ATP) and also a product of ATP metabolism. Thus as the brain uses stored energy in the form of ATP, adenosine builds up—and subjective sleepiness increases.<ref>[http://thebrain.mcgill.ca/flash/a/a_11/a_11_m/a_11_m_cyc/a_11_m_cyc.html Molecules that build up and make you sleep]. thebrain.mcgill.ca</ref> [[Caffeine]] and [[theophylline]] temporarily block the effect of adenosine, thus allowing it to build up further before the need for sleep reasserts itself.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, pp. 1113–1114. “Adenosine levels correlate with time spent awake. Endogenous, extracellular adenosine levels in the BF (102, 591, 843, 1017, 1018) and cortex (591, 1017) increase in proportion with time spent awake. Thus adenosine induces sleep and adenosine levels track sleep need, fulfilling the criteria for adenosine being a homeostatic sleep factor.”</ref> |
|||
=== |
=== Adult humans === |
||
[[File:Effects of sleep deprivation.svg|thumb|The main health effects of [[sleep deprivation]],<ref>Reference list is found on image page in Commons: [[:Commons:File:Effects of sleep deprivation.svg#References]]</ref> indicating impairment of normal maintenance by sleep.]] |
[[File:Effects of sleep deprivation.svg|thumb|The main health effects of [[sleep deprivation]],<ref>Reference list is found on image page in Commons: [[:Commons:File:Effects of sleep deprivation.svg#References]]</ref> indicating impairment of normal maintenance by sleep.]] |
||
| Line 87: | Line 131: | ||
Furthermore, sleep difficulties are closely associated with psychiatric disorders such as [[major depressive disorder|depression]], [[alcoholism]], and [[bipolar disorder]].<ref name="Thase2006">{{cite journal | author = Thase ME | title = Depression and sleep: pathophysiology and treatment | journal = Dialogues in clinical neuroscience | volume = 8 | issue = 2 | pages = 217–226 | year = 2006 | pmid = 16889107 | pmc = 3181772 | url = http://www.nlm.nih.gov/medlineplus/depression.html | issn = 1294-8322 | format = Free full text }}</ref> Up to 90% of adults with depression are found to have sleep difficulties. Dysregulation found on EEG includes disturbances in sleep continuity, decreased delta sleep and altered REM patterns with regard to latency, distribution across the night and density of eye movements.<ref>{{cite book |last=Mann |first=Joseph John |author2=David J. Kupfer |title=Biology of Depressive Disorders: Subtypes of depression and comorbid disorders, Part 2 |url=http://books.google.com/?id=qbbTmje6oskC&printsec=frontcover |format=Google books |accessdate=24 July 2009 |year=1993 |publisher=Springer |page=49 |isbn= 0-306-44296-5}}</ref> |
Furthermore, sleep difficulties are closely associated with psychiatric disorders such as [[major depressive disorder|depression]], [[alcoholism]], and [[bipolar disorder]].<ref name="Thase2006">{{cite journal | author = Thase ME | title = Depression and sleep: pathophysiology and treatment | journal = Dialogues in clinical neuroscience | volume = 8 | issue = 2 | pages = 217–226 | year = 2006 | pmid = 16889107 | pmc = 3181772 | url = http://www.nlm.nih.gov/medlineplus/depression.html | issn = 1294-8322 | format = Free full text }}</ref> Up to 90% of adults with depression are found to have sleep difficulties. Dysregulation found on EEG includes disturbances in sleep continuity, decreased delta sleep and altered REM patterns with regard to latency, distribution across the night and density of eye movements.<ref>{{cite book |last=Mann |first=Joseph John |author2=David J. Kupfer |title=Biology of Depressive Disorders: Subtypes of depression and comorbid disorders, Part 2 |url=http://books.google.com/?id=qbbTmje6oskC&printsec=frontcover |format=Google books |accessdate=24 July 2009 |year=1993 |publisher=Springer |page=49 |isbn= 0-306-44296-5}}</ref> |
||
=== |
=== Young humans === |
||
By the time infants reach the age of two, their brain size has reached 90 per cent of an adult-sized brain;<ref name=Dahl_2009>{{cite journal | author =Dahl RE | title = The regulation of sleep and arousal: Development and psychopathology | journal = Development and Psychopathology | year = 2009 | volume = 8 | issue =01 | pages = 3–27 | doi = 10.1017/S0954579400006945 }}</ref> a majority of this brain growth has occurred during the period of life with the highest rate of sleep. The hours that children spend asleep influence their ability to perform on cognitive tasks.<ref name = "Jenni_Dahl_2008" >{{cite book | editor = Nelson CA, Luciana M | title = Handbook of developmental cognitive neuroscience | year = 2008 | publisher = MIT Press | location = Cambridge, Mass. | isbn = 0262141043 | edition = 2nd | author = Jenni OG, Dahl RE | chapter = Sleep, cognition, and neuron, and emotion: A developmental review. | pages = 807–817 }}</ref><ref name="Scher_2005">{{cite journal | author = Scher A | title = Infant sleep at 10 months of age as a window to cognitive development | journal = Early Human Development | volume = 81 | issue = 3 | pages = 289–92 | year = 2005 | pmid = 15814211 | doi = 10.1016/j.earlhumdev.2004.07.005 }}</ref> Children who sleep through the night and have few night waking episodes have higher cognitive attainments and easier temperaments than other children.<ref name="Scher_2005"/><ref name="Spruyt_2007">{{cite journal | author = Spruyt K, Aitken RJ, So K, Charlton M, Adamson TM, Horne RS | title = Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life | journal = Early Human Development | volume = 84 | issue = 5 | pages = 289–96 | year = 2008 | pmid = 17707119 | doi = 10.1016/j.earlhumdev.2007.07.002 }}</ref><ref name="Bernier_2010">{{cite journal | author = Bernier A, Carlson SM, Bordeleau S, Carrier J | title = Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning | journal = Child Development | volume = 81 | issue = 6 | pages = 1739–52 | year = 2010 | pmid = 21077861 | doi = 10.1111/j.1467-8624.2010.01507.x }}</ref> |
By the time infants reach the age of two, their brain size has reached 90 per cent of an adult-sized brain;<ref name=Dahl_2009>{{cite journal | author =Dahl RE | title = The regulation of sleep and arousal: Development and psychopathology | journal = Development and Psychopathology | year = 2009 | volume = 8 | issue =01 | pages = 3–27 | doi = 10.1017/S0954579400006945 }}</ref> a majority of this brain growth has occurred during the period of life with the highest rate of sleep. The hours that children spend asleep influence their ability to perform on cognitive tasks.<ref name = "Jenni_Dahl_2008" >{{cite book | editor = Nelson CA, Luciana M | title = Handbook of developmental cognitive neuroscience | year = 2008 | publisher = MIT Press | location = Cambridge, Mass. | isbn = 0262141043 | edition = 2nd | author = Jenni OG, Dahl RE | chapter = Sleep, cognition, and neuron, and emotion: A developmental review. | pages = 807–817 }}</ref><ref name="Scher_2005">{{cite journal | author = Scher A | title = Infant sleep at 10 months of age as a window to cognitive development | journal = Early Human Development | volume = 81 | issue = 3 | pages = 289–92 | year = 2005 | pmid = 15814211 | doi = 10.1016/j.earlhumdev.2004.07.005 }}</ref> Children who sleep through the night and have few night waking episodes have higher cognitive attainments and easier temperaments than other children.<ref name="Scher_2005"/><ref name="Spruyt_2007">{{cite journal | author = Spruyt K, Aitken RJ, So K, Charlton M, Adamson TM, Horne RS | title = Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life | journal = Early Human Development | volume = 84 | issue = 5 | pages = 289–96 | year = 2008 | pmid = 17707119 | doi = 10.1016/j.earlhumdev.2007.07.002 }}</ref><ref name="Bernier_2010">{{cite journal | author = Bernier A, Carlson SM, Bordeleau S, Carrier J | title = Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning | journal = Child Development | volume = 81 | issue = 6 | pages = 1739–52 | year = 2010 | pmid = 21077861 | doi = 10.1111/j.1467-8624.2010.01507.x }}</ref> |
||
Sleep also influences language development. To test this, researchers taught infants a faux language and observed their recollection of the rules for that language.<ref name="Hupbach_2009">{{cite journal | author = Hupbach A, Gomez RL, Bootzin RR, Nadel L | title = Nap-dependent learning in infants | journal = Developmental Science | volume = 12 | issue = 6 | pages = 1007–12 | year = 2009 | pmid = 19840054 | doi = 10.1111/j.1467-7687.2009.00837.x | url = }}</ref> Infants who slept within four hours of learning the language could remember the language rules better, while infants who stayed awake longer did not recall those rules as well. There is also a relationship between infants' vocabulary and sleeping;<ref name="Bernier_2010"/> infants who sleep longer at night at 12 months have better vocabularies at 26 months.<ref name="Bernier_2010"/> |
Sleep also influences language development. To test this, researchers taught infants a faux language and observed their recollection of the rules for that language.<ref name="Hupbach_2009">{{cite journal | author = Hupbach A, Gomez RL, Bootzin RR, Nadel L | title = Nap-dependent learning in infants | journal = Developmental Science | volume = 12 | issue = 6 | pages = 1007–12 | year = 2009 | pmid = 19840054 | doi = 10.1111/j.1467-7687.2009.00837.x | url = }}</ref> Infants who slept within four hours of learning the language could remember the language rules better, while infants who stayed awake longer did not recall those rules as well. There is also a relationship between infants' vocabulary and sleeping;<ref name="Bernier_2010"/> infants who sleep longer at night at 12 months have better vocabularies at 26 months.<ref name="Bernier_2010"/> |
||
=== |
=== Recommendations === |
||
Children need many hours of sleep per day in order to develop and function properly: up to 18 hours for [[newborn]] babies, with a declining rate as a child ages.<ref name="autogenerated1"/> |
Children need many hours of sleep per day in order to develop and function properly: up to 18 hours for [[newborn]] babies, with a declining rate as a child ages.<ref name="autogenerated1"/> Early in 2015, after a two-year study,<ref name="sleepfoundation">{{cite journal | author= Hirshkowitz, Max ; Whiton, Kaitlyn et al. | title=National Sleep Foundation’s sleep time duration recommendations: methodology and results summary | journal= Sleep Health: Journal of the National Sleep Foundation |url= http://www.sleephealthjournal.org/article/S2352-7218(15)00015-7/fulltext |publisher= Elsevier Inc |date=14 January 2015 | pmid = | doi= 10.1016/j.sleh.2014.12.010 |accessdate=4 February 2015 }}</ref> the [[National Sleep Foundation]] in the US announced newly revised recommendations as shown in the table below. |
||
Early in 2015, after a two-year study,<ref name="sleepfoundation">{{cite journal | author= Hirshkowitz, Max ; Whiton, Kaitlyn et al. | title=National Sleep Foundation’s sleep time duration recommendations: methodology and results summary | journal= Sleep Health: Journal of the National Sleep Foundation |url= http://www.sleephealthjournal.org/article/S2352-7218(15)00015-7/fulltext |publisher= Elsevier Inc |date=14 January 2015 | pmid = | doi= 10.1016/j.sleh.2014.12.010 |accessdate=4 February 2015 }}</ref> the [[National Sleep Foundation]] in the US announced newly revised recommendations as shown in the table below. |
|||
{| class="wikitable" |
{| class="wikitable" |
||
| Line 123: | Line 166: | ||
| 7 to 9 hours<ref name="sleepfoundation" /> |
| 7 to 9 hours<ref name="sleepfoundation" /> |
||
|} |
|} |
||
==Naps== |
|||
[[File:People sleeping in a train.jpg|thumb|People sleeping on a train at night]] |
|||
{{main|Nap}} |
|||
The [[siesta]] habit has recently been associated with a 37% reduction in coronary mortality, possibly due to reduced cardiovascular stress mediated by daytime sleep.<ref name=Naska/> Nevertheless, [[Epidemiology|epidemiological]] studies on the relations between cardiovascular health and siestas have led to conflicting conclusions, possibly because of poor control of [[moderator variable]]s, such as physical activity. It is possible that people who take siestas have different physical activity habits, e.g., waking earlier and scheduling more activity during the morning. Such differences in physical activity may [[Mediator variable|mediate]] different 24-hour profiles in cardiovascular function. Even if such effects of physical activity can be discounted for explaining the relationship between siestas and cardiovascular health, it is still unknown whether it is the daytime nap itself, a [[Supine position|supine]] posture, or the expectancy of a nap that is the most important factor. It was recently suggested that a short nap can reduce stress and blood pressure (BP), with the main changes in BP occurring between the time of lights off and the onset of stage 1.<ref name=Zaregarizi1/><ref name=Zaregarizi2/> |
|||
Dr. Zaregarizi and his team have concluded that the acute time of falling asleep was when beneficial cardiovascular changes take place. This study has indicated that a large decline in BP occurs during the daytime sleep-onset period only when sleep is expected. However, when subjects rest in a [[supine position]], the same reduction in BP is not observed. This BP reduction may be associated with the lower coronary mortality rates seen in Mediterranean and Latin American populations in which siestas are common. |
|||
Dr. Zaregarizi assessed cardiovascular function (BP, heart rate, and measurements of blood vessel dilation) while nine healthy volunteers, 34 years of age on average, spent an hour standing quietly, reclining at rest but not sleeping, or reclining to nap. All participants were restricted to 4 hours of sleep on the night prior to each of the sleep laboratory tests. During the three phases of daytime sleep, he noted significant reductions in BP and heart rate. By contrast, they did not observe changes in cardiovascular function while the participants were standing or reclining at rest. |
|||
These findings also show that the greatest decline in BP occurs between lights-off and onset of daytime sleep itself. |
|||
During this sleep period, which lasted 9.7 minutes on average, BP decreased, while blood vessel dilation increased by more than 9 percent. |
|||
“There is little change in blood pressure once a subject is actually asleep," Dr. Zaregarizi noted, and he found minor changes in blood vessel dilation during sleep.<ref name=Zaregarizi1/><ref name=Zaregarizi2/> |
|||
Sleep duration in long-term experienced meditators is lower than in non-meditators and general population norms, with no apparent decrements in vigilance.<ref>{{cite journal | author = Kaul P, Passafiume J, Sargent CR, O'Hara BF | title = Meditation acutely improves psychomotor vigilance, and may decrease sleep need | journal = Behav Brain Funct | volume = 6 | pages = 47 | year = 2010 | pmid = 20670413 | pmc = 2919439 | doi = 10.1186/1744-9081-6-47 | doi_brokendate = 2015-02-02 }}</ref> |
|||
==Sleep debt== |
|||
{{Main|Sleep debt}} |
|||
Sleep debt is the effect of not getting enough sleep; a large debt causes mental, emotional and physical fatigue.{{citation needed|date=October 2013}} |
|||
Sleep debt results in diminished abilities to perform high-level cognitive functions. Neurophysiological and functional [[imaging studies]] have demonstrated that frontal regions of the brain are particularly responsive to homeostatic sleep pressure.<ref>{{cite journal | author = Gottselig JM, Adam M, Rétey JV, Khatami R, Achermann P, Landolt HP | title = Random number generation during sleep deprivation: effects of caffeine on response maintenance and stereotypy | journal = Journal of Sleep Research | volume = 15 | issue = 1 | pages = 31–40 | date = March 2006 | pmid = 16490000 | doi = 10.1111/j.1365-2869.2006.00497.x }}</ref> |
|||
Scientists do not agree on how much sleep debt it is possible to accumulate; whether it is accumulated against an individual's average sleep or some other benchmark; nor on whether the prevalence of sleep debt among adults has changed appreciably in the [[developed country|industrialized world]] in recent decades. It is likely that children are sleeping less than previously in [[Western world|Western societies]].<ref>{{cite journal | author = Iglowstein I, Jenni OG, Molinari L, Largo RH | title = Sleep duration from infancy to adolescence: reference values and generational trends | journal = Pediatrics | volume = 111 | issue = 2 | pages = 302–7 | date = February 2003 | pmid = 12563055 | doi = 10.1542/peds.111.2.302 | quote = Thus, the shift in the evening bedtime across cohorts accounted for the substantial decrease in sleep duration in younger children between the 1970s and the 1990s... [A] more liberal parental attitude toward evening bedtime in the past decades is most likely responsible for the bedtime shift and for the decline of sleep duration... }}</ref> |
|||
==Genetics== |
|||
It is hypothesized that a considerable amount of sleep-related behavior, such as when and how long a person needs to sleep, is regulated by genetics. Researchers have discovered some evidence that seems to support this assumption.<ref>{{cite journal | author = He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Rossner MJ, Nishino S, Fu YH | title = The Transcriptional Repressor DEC2 Regulates Sleep Length in Mammals | journal = Science | volume = 325 | issue = 5942 | pages = 866–70 | year = 2009 | pmid = 19679812 | pmc = 2884988 | doi = 10.1126/science.1174443 }}</ref> [[ABCC9]] is one gene found which influences the duration of human sleep.<ref name="The ABCC9 of Sleep: A Genetic Factor Regulates How Long We Sleep">{{cite web|title=The ABCC9 of Sleep: A Genetic Factor Regulates How Long We Sleep|url=http://www.sciencedaily.com/releases/2011/11/111124150237.htm|publisher=Science Daily|accessdate=21 August 2012}}</ref> |
|||
==Functions== |
==Functions== |
||
| Line 258: | Line 273: | ||
Early mammals engaged in polyphasic sleep, dividing sleep into multiple bouts per day. What then explains monophasic sleep behavior widely observed in mammals today? Higher daily sleep quotas and shorter sleep cycles in polyphasic species as compared to monophasic species, suggest that polyphasic sleep may be a less efficient means of attaining sleep’s benefits. Small species with higher BMR may therefore have less efficient sleep patterns. It follows that the evolution of monophasic sleep may hitherto be an unknown advantage of evolving larger mammalian body sizes and therefore lower BMR.<ref>{{cite journal | author = Capellini I, Nunn CL, McNamara P, Preston BT, Barton RA | title = Energetic constraints, not predation, influence the evolution of sleep patterning in mammals | journal = Functional Ecology | volume = 22 | issue = 5 | pages = 847–853 | year = 2008 | pmid = 20428321 | pmc = 2860325 | doi = 10.1111/j.1365-2435.2008.01449.x }}</ref> |
Early mammals engaged in polyphasic sleep, dividing sleep into multiple bouts per day. What then explains monophasic sleep behavior widely observed in mammals today? Higher daily sleep quotas and shorter sleep cycles in polyphasic species as compared to monophasic species, suggest that polyphasic sleep may be a less efficient means of attaining sleep’s benefits. Small species with higher BMR may therefore have less efficient sleep patterns. It follows that the evolution of monophasic sleep may hitherto be an unknown advantage of evolving larger mammalian body sizes and therefore lower BMR.<ref>{{cite journal | author = Capellini I, Nunn CL, McNamara P, Preston BT, Barton RA | title = Energetic constraints, not predation, influence the evolution of sleep patterning in mammals | journal = Functional Ecology | volume = 22 | issue = 5 | pages = 847–853 | year = 2008 | pmid = 20428321 | pmc = 2860325 | doi = 10.1111/j.1365-2435.2008.01449.x }}</ref> |
||
== |
==Genetics== |
||
It is hypothesized that a considerable amount of sleep-related behavior, such as when and how long a person needs to sleep, is regulated by genetics. Researchers have discovered some evidence that seems to support this assumption.<ref>{{cite journal | author = He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Rossner MJ, Nishino S, Fu YH | title = The Transcriptional Repressor DEC2 Regulates Sleep Length in Mammals | journal = Science | volume = 325 | issue = 5942 | pages = 866–70 | year = 2009 | pmid = 19679812 | pmc = 2884988 | doi = 10.1126/science.1174443 }}</ref> Monozygotic (identical) but not dizygotic (fraternal) twins tend to have similar sleep habits. Neurotransmitters, molecules whose production can be traced to specific genes, are one genetic influence on sleep which can be analyzed. And the circadian clock has its own set of genes.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, pp. 1138–1102.</ref> [[ABCC9]] is one gene found which influences the duration of human sleep.<ref name="The ABCC9 of Sleep: A Genetic Factor Regulates How Long We Sleep">{{cite web|title=The ABCC9 of Sleep: A Genetic Factor Regulates How Long We Sleep|url=http://www.sciencedaily.com/releases/2011/11/111124150237.htm|publisher=Science Daily|accessdate=21 August 2012}}</ref> |
|||
Insomnia, a dyssomnia, is a general term describing difficulty falling asleep and staying asleep. Insomnia can have many different causes, including psychological stress, a poor sleep environment, an inconsistent sleep schedule, or excessive mental or physical stimulation in the hours before bedtime. Insomnia is often treated through behavioral changes like keeping a regular sleep schedule, avoiding stimulating or stressful activities before bedtime, and cutting down on stimulants such as caffeine. The sleep environment may be improved by installing heavy drapes to shut out all sunlight, and keeping computers, televisions and work materials out of the sleeping area. |
|||
==Insomnia== |
|||
A 2010 review of published scientific research suggested that exercise generally improves sleep for most people, and helps sleep disorders such as insomnia. The optimum time to exercise ''may'' be 4 to 8 hours before bedtime, though exercise at any time of day is beneficial, with the exception of heavy exercise taken shortly before bedtime, which may disturb sleep. However there is insufficient evidence to draw detailed conclusions about the relationship between exercise and sleep.<ref>Buman, M.P. and King, A.C.: "Exercise as a Treatment to Enhance Sleep", ''[[American Journal of Lifestyle Medicine]]'', Nov–Dec 2010.</ref> |
|||
[[Insomnia]], a dyssomnia, is a general term describing difficulty falling asleep and staying asleep. Insomnia is the most common sleep problem, with many adults reporting occasional insomnia, and 10– 15% reporting a chronic condition.<ref>Brown ''et al.'' (2012), “Control of Sleep and Wakefulness”, pp. 1146–1147.</ref> Insomnia can have many different causes, including psychological stress, a poor sleep environment, an inconsistent sleep schedule, or excessive mental or physical stimulation in the hours before bedtime. Insomnia is often treated through behavioral changes like keeping a regular sleep schedule, avoiding stimulating or stressful activities before bedtime, and cutting down on stimulants such as caffeine. The sleep environment may be improved by installing heavy drapes to shut out all sunlight, and keeping computers, televisions and work materials out of the sleeping area. |
|||
Sleeping medications such as [[Ambien]] and [[Lunesta]] are an increasingly popular treatment for insomnia. Although these nonbenzodiazepine medications are generally believed to be better and safer than earlier generations of sedatives, they have still generated some controversy and discussion regarding side-effects. |
|||
[[White noise]] appears to be a promising treatment for [[insomnia]].<ref>{{cite journal | author = López HH, Bracha AS, Bracha HS | title = Evidence based complementary intervention for insomnia | journal = Hawaii Med J | volume = 61 | issue = 9 | pages = 192, 213 | year = 2002 | pmid = 12422383 | url = http://cogprints.org/5032/1/2002_H.M.J_White-noise_for_PTSD.pdf }}</ref> |
A 2010 review of published scientific research suggested that exercise generally improves sleep for most people, and helps sleep disorders such as insomnia. The optimum time to exercise ''may'' be 4 to 8 hours before bedtime, though exercise at any time of day is beneficial, with the exception of heavy exercise taken shortly before bedtime, which may disturb sleep. However there is insufficient evidence to draw detailed conclusions about the relationship between exercise and sleep.<ref>Buman, M.P. and King, A.C.: "Exercise as a Treatment to Enhance Sleep", ''[[American Journal of Lifestyle Medicine]]'', Nov–Dec 2010.</ref> Sleeping medications such as [[Ambien]] and [[Lunesta]] are an increasingly popular treatment for insomnia. Although these nonbenzodiazepine medications are generally believed to be better and safer than earlier generations of sedatives, they have still generated some controversy and discussion regarding side-effects. [[White noise]] appears to be a promising treatment for [[insomnia]].<ref>{{cite journal | author = López HH, Bracha AS, Bracha HS | title = Evidence based complementary intervention for insomnia | journal = Hawaii Med J | volume = 61 | issue = 9 | pages = 192, 213 | year = 2002 | pmid = 12422383 | url = http://cogprints.org/5032/1/2002_H.M.J_White-noise_for_PTSD.pdf }}</ref> |
||
==Obstructive sleep apnea== |
==Obstructive sleep apnea== |
||
| Line 356: | Line 370: | ||
File:Paul Klimsch Schlafender Jaguar.jpg|''Sleeping Jaguar'', {{Ill|de|Paul Klimsch}} |
File:Paul Klimsch Schlafender Jaguar.jpg|''Sleeping Jaguar'', {{Ill|de|Paul Klimsch}} |
||
File:Chrapek, the Sleepy one... (3734650615).jpg|''Shrapek'' (''Snorer''), [[Wrocław's dwarfs]] |
File:Chrapek, the Sleepy one... (3734650615).jpg|''Shrapek'' (''Snorer''), [[Wrocław's dwarfs]] |
||
File:Museo del Prado - Goya - Caprichos - No. 43 - El sueño de la razon produce monstruos.jpg|''[[The sleep of reason produces monsters]]'' by Goya, 1799 |
|||
</gallery> |
</gallery> |
||
| Line 399: | Line 414: | ||
<ref name=Zaregarizi2>{{cite book|title=Effects of Exercise & Daytime Sleep on Human Haemodynamics: With Focus on Changes in Cardiovascular Function during Daytime Sleep Onset|isbn=978-3-8484-1726-1|author=MohammadReza Zaregarizi}}</ref> |
<ref name=Zaregarizi2>{{cite book|title=Effects of Exercise & Daytime Sleep on Human Haemodynamics: With Focus on Changes in Cardiovascular Function during Daytime Sleep Onset|isbn=978-3-8484-1726-1|author=MohammadReza Zaregarizi}}</ref> |
||
}} |
}} |
||
=== Sources === |
|||
* Brown, Ritchie E., Radhika Basheer, James T. McKenna, Robert E. Strecker, & Robert W. McCarley (2012). “Control of Sleep and Wakefulness”. ''Physiological Review'' 92, pp. 1087–1187. |
|||
* Parmeggiani, Pier Luigi (2011). ''Systemic Homeostasis and Poikilostasis in Sleep: Is REM Sleep a Physiological Paradox?'' London: Imperial College Press. ISBN 978-1-94916-572-2 |
|||
==External links== |
==External links== |
||
Revision as of 15:15, 8 April 2015

Sleep is a naturally recurring state of animals characterized by altered consciousness, relatively inhibited sensory activity, and inhibition of nearly all voluntary muscles.[1] It is distinguished from wakefulness by a decreased ability to react to stimuli, but is more easily reversed than the state of hibernation or of being comatose. Mammalian sleep occurs in repeating periods, in which the body alternates between two highly distinct modes known as non-REM and REM sleep. REM stands for “rapid eye movement” but involves many other aspects including virtual paralysis of the body.
During sleep, most systems in an animal are in a heightened anabolic state, accentuating the growth and rejuvenation of the immune, nervous, skeletal, and muscular systems. Sleep in non-human animals is observed in mammals, birds, reptiles, amphibians, and fish, and in some form in insects and even in simpler animals such as nematodes. The internal circadian clock tends to promote sleep during a regular time of day or night. However, sleep patterns vary widely among animals and within the human species. Industrialization and artificial light have substantially altered human sleep habits.
The diverse purposes and mechanisms of sleep are the subject of substantial ongoing research.[2] Sleep seems to assist animals with improvements in the body and mind. A well-known feature of sleep in humans is the dream, an experience typically recounted in narrative form, which resembles waking life while in progress, but which usually can later be distinguished as fantasy. Humans may suffer from a number of sleep disorders. These include dyssomnias (such as insomnia, hypersomnia, and sleep apnea), parasomnias (such as sleepwalking and REM behavior disorder), and the circadian rhythm sleep disorders.
Physiology

In mammals and birds, sleep is divided into two broad types: rapid eye movement (REM sleep) and non-rapid eye movement (NREM or non-REM sleep). Each type has a distinct set of physiological and neurological features associated with it. REM sleep is associated with dreaming, desynchronized and faster brain waves, loss of muscle tone, and suspension of homeostasis.[3] REM and non-REM sleep are so different that physiologists classify them as distinct behavioral states. In this view, REM, non-REM, and waking represent the three major modes of consciousness, neural activity, and physiological regulation.[4] According to the Hobson & McCarley activation-synthesis hypothesis, proposed in 1975–1977, the alternation between REM and non-REM can be explained in terms of cycling, reciprocally influential neurotransmitter systems.[5]
Especially during non-REM sleep, the brain uses significantly less energy during sleep than it does in waking. In areas with reduced activity, the brain restores its supply of adenosine triphosphate (ATP), the molecule used for short-term storage and transport of energy.[6]
Key physiological indicators in sleep include EEG of brain waves, electrooculography (EOG) of eye movements, and electromyography (EMG) of skeletal muscle activity. Simultaneous collection of these measurements is called polysomnography and can be performed in a specialized sleep laboratory.[7]
Stages
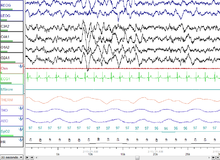

Human sleep occurs in periods of approximately 90 minutes, which include an increasing proportion of paradoxical (REM) sleep as they repeat. This rhythm is called the ultradian sleep cycle.[8] Sleep proceeds in cycles of REM and NREM, usually four or five of them per night. The American Academy of Sleep Medicine (AASM) divides NREM into three stages: N1, N2, and N3, the last of which is also called delta sleep or slow-wave sleep.[9] The whole period normally proceeds in the order: N1 → N2 → N3 → N2 → REM. In other animals the subdivision between phases of non-REM sleep is not typically used, although animal non-REM sleep can be described as ligher or deeper.[10] There is a greater amount of deep sleep (stage N3) earlier in the night, while the proportion of REM sleep increases in the two cycles just before natural awakening.
Each stage may have a distinct physiological function and this can result in sleep that exhibits loss of consciousness but does not fulfill its physiological functions (i.e., one may still feel tired after apparently sufficient sleep).
Non-REM
As an awake organism falls asleep, the activity of its body slows down. Body temperature, heart rate, breathing rate, and energy use all decrease. Brain waves get slower and bigger. The excitatory neurotransmitter acetylcholine becomes less available in the brain.[11] The organism will maneuver, as best it can, to create a thermally friendly environment—for example, by curling up into a ball if it's cold out. Reflexes remain fairly active. These characteristics apply to some degree during all non-REM sleep, which constitutes ~80% of all sleep in humans.[12]
NREM 1
NREM stage 1 (N1): This is a stage of sleep that usually occurs between sleep and wakefulness, and sometimes occurs between periods of deeper sleep and periods of REM. The muscles are active, and the eyes roll slowly, opening and closing moderately. The brain transitions from alpha waves having a frequency of 8–13 Hz (common in the awake state) to theta waves having a frequency of 4–7 Hz. This stage is sometimes referred to as somnolence or drowsy sleep. Sudden twitches and hypnic jerks, also known as positive myoclonus, may be associated with the onset of sleep during N1. Some people may also experience hypnagogic hallucinations during this stage. During N1, the organism loses some muscle tone and most conscious awareness of the external environment.
NREM 2
NREM stage 2 (N2): In this stage, theta activity is observed and sleepers become gradually harder to awaken; the alpha waves of the previous stage are interrupted by abrupt activity called sleep spindles (or thalamocortical spindles) and K-complexes.[13] Sleep spindles range from 11 to 16 Hz (most commonly 12–14 Hz) and K-complexes. During this stage, muscular activity as measured by EMG decreases, and conscious awareness of the external environment disappears. This stage occupies 45–55% of total sleep in adults.
NREM 3
NREM stage 3: Formerly divided into stages 3 and 4, this stage is called slow-wave sleep (SWS) or deep sleep. SWS is initiated in the preoptic area and consists of delta activity, high amplitude waves at less than 3.5 Hz. The sleeper is less responsive to the environment; many environmental stimuli no longer produce any reactions.
This stage is characterized by the presence of a minimum of 20% delta waves ranging from 0.5–2 Hz and having a peak-to-peak amplitude >75 μV. (EEG standards define delta waves to be from 0 to 4 Hz, but sleep standards in both the original R&K, as well as the new 2007 AASM guidelines have a range of 0.5–2 Hz.) This is the stage in which parasomnias such as night terrors, nocturnal enuresis, sleepwalking, and somniloquy occur. Many illustrations and descriptions still show a stage N3 with 20–50% delta waves and a stage N4 with greater than 50% delta waves; these have been combined as stage N3.
REM
REM: The sleeper now enters rapid eye movement (REM) where most muscles are paralyzed. REM sleep is turned on by acetylcholine secretion and is inhibited by neurons that secrete serotonin. This level is also referred to as paradoxical sleep because the sleeper, although exhibiting EEG waves similar to a waking state, is harder to arouse than at any other sleep stage. Vital signs indicate arousal and oxygen consumption by the brain is higher than when the sleeper is awake.[14] An adult reaches REM approximately every 90 minutes, with the latter half of sleep being more dominated by this stage. REM sleep occurs as a person returns to stage 1 from a deep sleep.[3] The function of REM sleep is uncertain but a lack of it impairs the ability to learn complex tasks. One approach to understanding the role of sleep is to study the deprivation of it.[15] During periods of REM the EEG pattern returns to high-frequency waves that look similar to the waves produced while the person is awake.[13]
Rapid eye movement sleep, or REM sleep (also known as paradoxical sleep),[16] accounts for 20–25% of total sleep time in most human adults. The criteria for REM sleep include rapid eye movements as well as a rapid low-voltage EEG. During REM sleep, EEG patterns returns to higher frequency saw-tooth waves. Most memorable dreaming occurs in this stage. At least in mammals, a descending muscular atonia is seen. Such paralysis may be necessary to protect organisms from self-damage through physically acting out scenes from the often-vivid dreams that occur during this stage.[citation needed]
A newborn baby spends almost 9 hours a day just in REM sleep. By the age of five or so, only slightly over two hours is spent in REM.[17]
Awakening
Scientific studies on sleep have shown that sleep stage at awakening is an important factor in amplifying sleep inertia.[citation needed] Alarm clocks involving sleep stage monitoring appeared on the market in 2005.[18] Using sensing technologies such as EEG electrodes or accelerometers, these alarm clocks are supposed to wake people only from light sleep.
Historical development of stages model
The stages of sleep were first described in 1937 by Alfred Lee Loomis and his coworkers, who separated the different electroencephalography (EEG) features of sleep into five levels (A to E), which represented the spectrum from wakefulness to deep sleep.[19] In 1953, REM sleep was discovered as distinct, and thus William Dement and Nathaniel Kleitman reclassified sleep into four NREM stages and REM.[20] The staging criteria were standardized in 1968 by Allan Rechtschaffen and Anthony Kales in the "R&K sleep scoring manual."[21][10]
In the R&K standard, NREM sleep was divided into four stages, with slow-wave sleep comprising stages 3 and 4. In stage 3, delta waves made up less than 50% of the total wave patterns, while they made up more than 50% in stage 4. Furthermore, REM sleep was sometimes referred to as stage 5. In 2004, the AASM commissioned the AASM Visual Scoring Task Force to review the R&K scoring system. The review resulted in several changes, the most significant being the combination of stages 3 and 4 into Stage N3. The revised scoring was published in 2007 as The AASM Manual for the Scoring of Sleep and Associated Events.[22] Arousals and respiratory, cardiac, and movement events were also added.[23][24]
Circadian timing
Sleep timing is controlled by the circadian clock, sleep-wake homeostasis, and in humans, within certain bounds, willed behavior. The circadian clock—an inner timekeeping, temperature-fluctuating, enzyme-controlling device—works in tandem with these other mechanisms. Circadian timing, known as process C, is cyclical, based on the time of day; sleep-wake homeostasis, or process S, operates on a more absolute scale.
Circadian clock
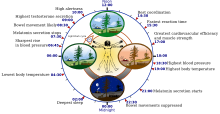
In diurnal animals, sleepiness occurs as the circadian element causes the release of the hormone melatonin and a gradual decrease in core body temperature. The timing is affected by one's chronotype. It is the circadian rhythm that determines the ideal timing of a correctly structured and restorative sleep episode.[25]
Distribution
In polyphasic sleep, an organism sleeps at multiple times during a 24-hour cycle. Monophasic sleep occurs all at once.
Naps
The siesta habit has recently been associated with a 37% reduction in coronary mortality, possibly due to reduced cardiovascular stress mediated by daytime sleep.[26] Nevertheless, epidemiological studies on the relations between cardiovascular health and siestas have led to conflicting conclusions, possibly because of poor control of moderator variables, such as physical activity. It is possible that people who take siestas have different physical activity habits, e.g., waking earlier and scheduling more activity during the morning. Such differences in physical activity may mediate different 24-hour profiles in cardiovascular function. Even if such effects of physical activity can be discounted for explaining the relationship between siestas and cardiovascular health, it is still unknown whether it is the daytime nap itself, a supine posture, or the expectancy of a nap that is the most important factor. It was recently suggested that a short nap can reduce stress and blood pressure (BP), with the main changes in BP occurring between the time of lights off and the onset of stage 1.[27][28]
Dr. Zaregarizi and his team have concluded that the acute time of falling asleep was when beneficial cardiovascular changes take place. This study has indicated that a large decline in BP occurs during the daytime sleep-onset period only when sleep is expected. However, when subjects rest in a supine position, the same reduction in BP is not observed. This BP reduction may be associated with the lower coronary mortality rates seen in Mediterranean and Latin American populations in which siestas are common. Dr. Zaregarizi assessed cardiovascular function (BP, heart rate, and measurements of blood vessel dilation) while nine healthy volunteers, 34 years of age on average, spent an hour standing quietly, reclining at rest but not sleeping, or reclining to nap. All participants were restricted to 4 hours of sleep on the night prior to each of the sleep laboratory tests. During the three phases of daytime sleep, he noted significant reductions in BP and heart rate. By contrast, they did not observe changes in cardiovascular function while the participants were standing or reclining at rest. These findings also show that the greatest decline in BP occurs between lights-off and onset of daytime sleep itself.
During this sleep period, which lasted 9.7 minutes on average, BP decreased, while blood vessel dilation increased by more than 9 percent.
“There is little change in blood pressure once a subject is actually asleep," Dr. Zaregarizi noted, and he found minor changes in blood vessel dilation during sleep.[27][28]
Sleep duration in long-term experienced meditators is lower than in non-meditators and general population norms, with no apparent decrements in vigilance.[29]
Sleep homeostasis, deprivation and optimization
Generally speaking, the more an organism is awake, the more it feels a need to sleep. The balance between sleeping and waking is called homeostasis. Induced or perceived lack of sleep is commonly called sleep deprivation.
Sleep deprivation tends to cause slower brain waves in the frontal cortex, shortened attention span, higher anxiety, impaired memory, and a grouchy mood. Conversely, a well-rested organism tends to have improved memory and mood.[30]
Duration
Homeostatic sleep propensity (the need for sleep as a function of the amount of time elapsed since the last adequate sleep episode) must be balanced against the circadian element for satisfactory sleep.[31] Along with corresponding messages from the circadian clock, this tells the body it needs to sleep.[32] Sleep offset (awakening) is primarily determined by circadian rhythm. A person who regularly awakens at an early hour will generally not be able to sleep much later than his or her normal waking time, even if moderately sleep-deprived[citation needed].
Sleep duration is affected by the gene DEC2. People with a certain DEC2 mutation sleep two hours less than normal. The gene also affects the sleep patterns of mice, and likely does so for all mammals.[33][34]
Sleep debt
Sleep debt is the effect of not getting enough sleep; a large debt causes mental, emotional and physical fatigue.[citation needed]
Sleep debt results in diminished abilities to perform high-level cognitive functions. Neurophysiological and functional imaging studies have demonstrated that frontal regions of the brain are particularly responsive to homeostatic sleep pressure.[35]
Scientists do not agree on how much sleep debt it is possible to accumulate; whether it is accumulated against an individual's average sleep or some other benchmark; nor on whether the prevalence of sleep debt among adults has changed appreciably in the industrialized world in recent decades. It is likely that children are sleeping less than previously in Western societies.[36]
One neurochemical indicator of sleep debt is adenosine, a neurotransmitter that inhibits many of the bodily processes associated with wakefulness. Adenosine is an ingredient in adenosine triphosphate (ATP) and also a product of ATP metabolism. Thus as the brain uses stored energy in the form of ATP, adenosine builds up—and subjective sleepiness increases.[37] Caffeine and theophylline temporarily block the effect of adenosine, thus allowing it to build up further before the need for sleep reasserts itself.[38]
Adult humans

The optimal amount of sleep is not a meaningful concept unless the timing of that sleep is seen in relation to an individual's circadian rhythms. A person's major sleep episode is relatively inefficient and inadequate when it occurs at the "wrong" time of day; one should be asleep at least six hours before the lowest body temperature.[40] The timing is correct when the following two circadian markers occur after the middle of the sleep episode and before awakening:[41] maximum concentration of the hormone melatonin, and minimum core body temperature.
Human sleep needs vary by age and amongst individuals, and sleep is considered to be adequate when there is no daytime sleepiness or dysfunction. Moreover, self-reported sleep duration is only moderately correlated with actual sleep time as measured by actigraphy,[42] and those affected with sleep state misperception may typically report having slept only four hours despite having slept a full eight hours.[43]
A University of California, San Diego psychiatry study of more than one million adults found that people who live the longest self-report sleeping for six to seven hours each night.[44] Another study of sleep duration and mortality risk in women showed similar results.[45] Other studies show that "sleeping more than 7 to 8 hours per day has been consistently associated with increased mortality," though this study suggests the cause is probably other factors such as depression and socioeconomic status, which would correlate statistically.[46] It has been suggested that the correlation between lower sleep hours and reduced morbidity only occurs with those who wake naturally, rather than those who use an alarm. [citation needed]
Researchers at the University of Warwick and University College London have found that lack of sleep can more than double the risk of death from cardiovascular disease, but that too much sleep can also be associated with a doubling of the risk of death, though not primarily from cardiovascular disease.[47]
Professor Francesco Cappuccio said, "Short sleep has been shown to be a risk factor for weight gain, hypertension, and Type 2 diabetes, sometimes leading to mortality; but in contrast to the short sleep-mortality association, it appears that no potential mechanisms by which long sleep could be associated with increased mortality have yet been investigated. Some candidate causes for this include depression, low socioeconomic status, and cancer-related fatigue... In terms of prevention, our findings indicate that consistently sleeping around seven hours per night is optimal for health, and a sustained reduction may predispose to ill health."[clarification needed]
Furthermore, sleep difficulties are closely associated with psychiatric disorders such as depression, alcoholism, and bipolar disorder.[48] Up to 90% of adults with depression are found to have sleep difficulties. Dysregulation found on EEG includes disturbances in sleep continuity, decreased delta sleep and altered REM patterns with regard to latency, distribution across the night and density of eye movements.[49]
Young humans
By the time infants reach the age of two, their brain size has reached 90 per cent of an adult-sized brain;[50] a majority of this brain growth has occurred during the period of life with the highest rate of sleep. The hours that children spend asleep influence their ability to perform on cognitive tasks.[51][52] Children who sleep through the night and have few night waking episodes have higher cognitive attainments and easier temperaments than other children.[52][53][54]
Sleep also influences language development. To test this, researchers taught infants a faux language and observed their recollection of the rules for that language.[55] Infants who slept within four hours of learning the language could remember the language rules better, while infants who stayed awake longer did not recall those rules as well. There is also a relationship between infants' vocabulary and sleeping;[54] infants who sleep longer at night at 12 months have better vocabularies at 26 months.[54]
Recommendations
Children need many hours of sleep per day in order to develop and function properly: up to 18 hours for newborn babies, with a declining rate as a child ages.[32] Early in 2015, after a two-year study,[56] the National Sleep Foundation in the US announced newly revised recommendations as shown in the table below.
| Age and condition | Sleep Needs |
|---|---|
| Newborns (0–3 months) | 14 to 17 hours[56] |
| Infants (4–11 months) | 12 to 15 hours[56] |
| Toddlers (1–2 years) | 11 to 14 hours[56] |
| Preschoolers (3–5 years) | 10 to 13 hours[56] |
| School-age children (6–13 years) | 9 to 11 hours[56] |
| Teenagers (14–17 years) | 8 to 10 hours[56][57] |
| Adults | 7 to 9 hours[56] |
Functions
The multiple hypotheses proposed to explain the function of sleep reflect the incomplete understanding of the subject. (When asked, after 50 years of research, what he knew about the reason people sleep, William Dement, founder of Stanford University's Sleep Research Center, answered, "As far as I know, the only reason we need to sleep that is really, really solid is because we get sleepy.")[58] It is likely that sleep evolved to fulfill some primeval function and took on multiple functions over time[citation needed] (analogous to the larynx, which controls the passage of food and air, but descended over time to develop speech capabilities).
If sleep were not essential, one would expect to find:
- Animal species that do not sleep at all
- Animals that do not need recovery sleep after staying awake longer than usual
- Animals that suffer no serious consequences as a result of lack of sleep
Outside of a few basal animals that have no brain or a very simple one, no animals have been found to date that satisfy any of these criteria.[59] While some varieties of shark, such as great whites and hammerheads, must remain in motion at all times to move oxygenated water over their gills, it is possible they still sleep one cerebral hemisphere at a time as marine mammals do. However it remains to be shown definitively whether any fish is capable of unihemispheric sleep.
Sleep is sometimes thought to help conserve energy, though this theory is not fully adequate as it only decreases metabolism by about 5–10%.[60][61] Additionally it is observed that mammals require sleep even during the hypometabolic state of hibernation, in which circumstance it is actually a net loss of energy as the animal returns from hypothermia to euthermia in order to sleep.[62]
Some of the many proposed functions of sleep are as follows:
Increased waste clearance of brain
A publication by L. Xie and colleagues in 2013 explored the efficiency of the glymphatic system during sleep and provided the first direct evidence that the clearance of interstitial waste products increases during the resting state. Using a combination of diffusion ionophoresis techniques pioneered by Nicholson and colleagues, in vivo 2-photon imaging, and electroencephalography to confirm the wake and sleep states, Xia and Nedergaard demonstrated that the changes in efficiency of CSF–ISF exchange between the awake and sleeping brain were caused by expansion and contraction of the extracellular space, which increased by ~60% in the sleeping brain to promote clearance of interstitial wastes such as amyloid beta.[63] On the basis of these findings, they hypothesized that the restorative properties of sleep may be linked to increased glymphatic clearance of metabolic waste products produced by neural activity in the awake brain.
Restoration
Wound healing has been shown to be affected by sleep. Sleep deprivation hinders the healing of burns on rats.[64]
It has been shown that sleep deprivation affects the immune system. When compared with a control group, sleep-deprived rats' blood tests indicated a 20% decrease in white blood cell count, a significant change in the immune system.[65] It is now possible to state that "sleep loss impairs immune function and immune challenge alters sleep," and it has been suggested that mammalian species which invest in longer sleep times are investing in the immune system, as species with the longer sleep times have higher white blood cell counts.[66] A 2014 study found that depriving mice of sleep increased cancer growth and dampened the immune system's ability to control cancers. The researchers found higher levels of M2 tumor-associated macrophages and TLR4 molecules in the sleep deprived mice and proposed this as the mechanism for increased susceptibility of the mice to cancer growth. M2 cells suppress the immune system and encourage tumour growth. TRL4 molecules are signalling molecules in the activation of the immune system.[67] Sleep has also been theorized to effectively combat the accumulation of free radicals in the brain, by increasing the efficiency of endogeneous antioxidant mechanisms.[68]
The effect of sleep duration on somatic growth is not completely known. One study recorded growth, height, and weight, as correlated to parent-reported time in bed in 305 children over a period of nine years (age 1–10). It was found that "the variation of sleep duration among children does not seem to have an effect on growth."[69] It has been shown that sleep—more specifically, slow-wave sleep (SWS)—does affect growth hormone levels in adult men. During eight hours' sleep, Van Cauter, Leproult, and Plat[70] found that the men with a high percentage of SWS (average 24%) also had high growth hormone secretion, while subjects with a low percentage of SWS (average 9%) had low growth hormone secretion.
There is some supporting evidence of the restorative function of sleep. The sleeping brain has been shown to remove metabolic waste products at a faster rate than during an awake state.[71] While awake, metabolism generates reactive oxygen species, which are damaging to cells. In sleep, metabolic rates decrease and reactive oxygen species generation is reduced allowing restorative processes to take over. It is theorized that sleep helps facilitate the synthesis of molecules that help repair and protect the brain from these harmful elements generated during waking.[72] The metabolic phase during sleep is anabolic; anabolic hormones such as growth hormones (as mentioned above) are secreted preferentially during sleep. The duration of sleep among species is, broadly speaking, inversely related to animal size[citation needed] and directly related to basal metabolic rate (BMR). Rats, which have a high BMR, sleep for up to 14 hours a day, whereas elephants and giraffes, which have lower BMRs, sleep only 3–4 hours per day.
Energy conservation could as well have been accomplished by resting quiescent without shutting off the organism from the environment, potentially a dangerous situation. A sedentary nonsleeping animal is more likely to survive predators, while still preserving energy. Sleep, therefore, seems to serve another purpose, or other purposes, than simply conserving energy; for example, hibernating animals waking up from hibernation go into rebound sleep because of lack of sleep during the hibernation period. They are definitely well-rested and are conserving energy during hibernation, but need sleep for something else.[62] Rats kept awake indefinitely develop skin lesions, hyperphagia, loss of body mass, hypothermia, and, eventually, fatal sepsis.[73]
Another potential purpose for sleep could be to restore signal strength in synapses that are activated while awake to a "baseline" level, weakening unnecessary connections to better facilitate learning and memory functions again the next day.[74]
Ontogenesis
According to the ontogenetic hypothesis of REM sleep, the activity occurring during neonatal REM sleep (or active sleep) seems to be particularly important to the developing organism.[75] Studies investigating the effects of deprivation of active sleep have shown that deprivation early in life can result in behavioral problems, permanent sleep disruption, decreased brain mass,[76] and an abnormal amount of neuronal cell death.[77]
REM sleep appears to be important for development of the brain. REM sleep occupies the majority of time of sleep of infants, who spend most of their time sleeping. Among different species, the more immature the baby is born, the more time it spends in REM sleep. Proponents also suggest that REM-induced muscle inhibition in the presence of brain activation exists to allow for brain development by activating the synapses, yet without any motor consequences that may get the infant in trouble. Additionally, REM deprivation results in developmental abnormalities later in life.
However, this does not explain why older adults still need REM sleep. Aquatic mammal infants do not have REM sleep in infancy;[78] REM sleep in those animals increases as they age.
Memory processing
Scientists have shown numerous ways in which sleep is related to memory. In a study conducted by Turner, Drummond, Salamat, and Brown (2007),[79] working memory was shown to be affected by sleep deprivation. Working memory is important because it keeps information active for further processing and supports higher-level cognitive functions such as decision making, reasoning, and episodic memory. The study allowed 18 women and 22 men to sleep only 26 minutes per night over a four-day period. Subjects were given initial cognitive tests while well-rested, and then were tested again twice a day during the four days of sleep deprivation. On the final test, the average working memory span of the sleep-deprived group had dropped by 38% in comparison to the control group.
The relation between working memory and sleep can also be explored by testing how working memory works during sleep. Daltrozzo, Claude, Tillmann, Bastuji, and Perrin,[80] using Event-Related Potentials to the perception of sentences during sleep showed that working memory for linguistic information is partially preserved during sleep with a smaller capacity compared to wake.
Memory seems to be affected differently by certain stages of sleep such as REM and slow-wave sleep (SWS). In one study,[81] multiple groups of human subjects were used: wake control groups and sleep test groups. Sleep and wake groups were taught a task and were then tested on it, both on early and late nights, with the order of nights balanced across participants. When the subjects' brains were scanned during sleep, hypnograms revealed that SWS was the dominant sleep stage during the early night, representing around 23% on average for sleep stage activity.
The early-night test group performed 16% better on the declarative memory test than the control group. During late-night sleep, REM became the most active sleep stage at about 24%, and the late-night test group performed 25% better on the procedural memory test than the control group. This suggests that procedural memory benefits from late, REM-rich sleep, whereas declarative memory benefits from early, slow wave-rich sleep.
A study conducted by Datta[82] indirectly supports these results. The subjects chosen were 22 male rats. A box was constructed wherein a single rat could move freely from one end to the other. The bottom of the box was made of a steel grate. A light would shine in the box accompanied by a sound. After a five-second delay, an electrical shock would be applied. Once the shock commenced, the rat could move to the other end of the box, ending the shock immediately. The rat could also use the five-second delay to move to the other end of the box and avoid the shock entirely.
The length of the shock never exceeded five seconds. This was repeated 30 times for half the rats. The other half, the control group, was placed in the same trial, but the rats were shocked regardless of their reaction. After each of the training sessions, the rat would be placed in a recording cage for six hours of polygraphic recordings.
This process was repeated for three consecutive days. This study found that during the posttrial sleep recording session, rats spent 25.47% more time in REM sleep after learning trials than after control trials. These trials support the results of the Born et al. study, suggesting a correlation between REM sleep and procedural knowledge.
An observation of the Datta study is that the learning group spent 180% more time in SWS than did the control group during the post-trial sleep-recording session.[83] This study shows that after spatial exploration activity, patterns of hippocampal place cells are reactivated during SWS following the experiment. Rats were run through a linear track using rewards on either end. The rats would then be placed in the track for 30 minutes to allow them to adjust (PRE), then they ran the track with reward-based training for 30 minutes (RUN), and then they were allowed to rest for 30 minutes.
During each of these three periods, EEG data were collected for information on the rats' sleep stages. The mean firing rates of hippocampal place cells during prebehavior SWS (PRE) and three ten-minute intervals in postbehavior SWS (POST) were calculated by averaging across 22 track-running sessions from seven rats. The results showed that ten minutes after the trial RUN session, there was a 12% increase in the mean firing rate of hippocampal place cells from the PRE level. After 20 minutes, the mean firing rate returned rapidly toward the PRE level. The elevated firing of hippocampal place cells during SWS after spatial exploration could explain why there were elevated levels of slow-wave sleep in Datta's study, as it also dealt with a form of spatial exploration.
A study has also been done involving direct current stimulation to the prefrontal cortex to increase the amount of slow oscillations during SWS. The direct current stimulation greatly enhanced word-pair retention the following day, giving evidence that SWS plays a large role in the consolidation of episodic memories.[84]
The different studies suggest that there is a correlation between sleep and the complex functions of memory. Harvard sleep researchers Saper[85] and Stickgold[86] point out that an essential part of memory and learning consists of nerve cell dendrites' sending of information to the cell body to be organized into new neuronal connections. This process demands that no external information is presented to these dendrites, and it is suggested that this may be why it is during sleep that memories and knowledge are solidified and organized.
Recent studies examining gene expression and evolutionary increases in brain size offer complimentary support for the role of sleep in the mammalian memory consolidation theory. Evolutionary advances in the size of the mammalian amygdala, (a brain structure active during sleep and involved in memory processing), are also associated with increases in NREM sleep durations.[87] Likewise, nighttime gene expression differs from daytime expression and specifically targets genes thought to be involved in memory consolidation and brain plasticity.[88]
Preservation
The "Preservation and Protection" theory holds that sleep serves an adaptive function. It protects the animal during that portion of the 24-hour day in which being awake, and hence roaming around, would place the individual at greatest risk.[89] Organisms do not require 24 hours to feed themselves and meet other necessities. From this perspective of adaptation, organisms are safer by staying out of harm's way, where potentially they could be prey to other, stronger organisms. They sleep at times that maximize their safety, given their physical capacities and their habitats.
This theory fails to explain why the brain disengages from the external environment during normal sleep. However, the brain consumes a large proportion of the body's energy at any one time and preservation of energy could only occur by limiting its sensory inputs. Another argument against the theory is that sleep is not simply a passive consequence of removing the animal from the environment, but is a "drive"; animals alter their behaviors in order to obtain sleep.
Therefore, circadian regulation is more than sufficient to explain periods of activity and quiescence that are adaptive to an organism, but the more peculiar specializations of sleep probably serve different and unknown functions. Moreover, the preservation theory needs to explain why carnivores like lions, which are on top of the food chain and thus have little to fear, sleep the most. It has been suggested that they need to minimize energy expenditure when not hunting.
Preservation also does not explain why aquatic mammals sleep while moving. Quiescence during these vulnerable hours would do the same and would be more advantageous, because the animal would still be able to respond to environmental challenges like predators, etc. Sleep rebound that occurs after a sleepless night will be maladaptive, but obviously must occur for a reason. A zebra falling asleep the day after it spent the sleeping time running from a lion is more, not less, vulnerable to predation.
Emotional impacts
Some research shows sleep clears negative emotions.[90]
Dreaming

Dreaming is the perceived experience of sensory images and sounds during sleep, in a sequence which the dreamer usually perceives more as an apparent participant than as an observer. Dreaming is stimulated by the pons and mostly occurs during the REM phase of sleep.
As Dement studied, he found out that people need REM, or dreaming, sleep. He conducted a sleep and dream research project, in which the first eight of his participants were published in the article he wrote. All eight were male. For a maximum span of a 7 days, he varyingly deprived the participants of strictly REM sleep by waking them each time they started to enter the stage. He monitored this with small electrodes attached to their scalp and temples. As the study went on, he noticed that the more he deprived them of REM sleep, the more often he had to wake the men. Afterwards, they showed more REM sleep than usual.[91][92]
Dreams can also be suppressed or encouraged; using anti-depressants, acetaminophen, ibuprofen, or alcoholic beverages is thought to potentially suppress dreams, whereas melatonin may have the ability to encourage them.[93]
People have proposed many hypotheses about the functions of dreaming. Sigmund Freud postulated that dreams are the symbolic expression of frustrated desires that have been relegated to the unconscious mind, and he used dream interpretation in the form of psychoanalysis to uncover these desires. See Freud: The Interpretation of Dreams.
While penile erections during sleep are commonly believed to indicate dreams with sexual content, they are not more frequent during sexual dreams than they are during nonsexual dreams.[94] The parasympathetic nervous system experiences increased activity during REM sleep which may cause erection of the penis or clitoris. In males, 80% to 95% of REM sleep is normally accompanied by partial to full penile erection, while only about 12% of men's dreams contain sexual content.[14]
Freud's work concerns the psychological role of dreams, which does not exclude any physiological role they may have. Recent research claims that sleep has the overall role of consolidation and organization of synaptic connections formed during learning and experience.[95] As such, Freud's work is not ruled out. Nevertheless, Freud's research has been expanded on, especially with regard to the organization and consolidation of recent memory.
Certain processes in the cerebral cortex have been studied by John Allan Hobson and Robert McCarley. In their activation synthesis theory, for example, they propose that dreams are caused by the random firing of neurons in the cerebral cortex during the REM period. Neatly, this theory helps explain the irrationality of the mind during REM periods, as, according to this theory, the forebrain then creates a story in an attempt to reconcile and make sense of the nonsensical sensory information presented to it.[96] Ergo, the odd nature of many dreams.
Evolution
According to Tsoukalas (2012) REM sleep is an evolutionary transformation of a well-known defensive mechanism, the tonic immobility reflex. This reflex, also known as animal hypnosis or death feigning, functions as the last line of defense against an attacking predator and consists of the total immobilization of the animal: the animal appears dead (cf. “playing possum”). The neurophysiology and phenomenology of this reaction shows striking similarities to REM sleep, a fact which betrays a deep evolutionary kinship. For example, both reactions exhibit brainstem control, paralysis, sympathetic activation, and thermoregulatory changes. This theory integrates many earlier findings into a unified, and evolutionary well informed, framework.[97][98]
Mammals, birds and reptiles evolved from amniotic ancestors, the first vertebrates with life cycles independent of water. The fact that birds and mammals are the only known animals to exhibit REM and NREM sleep indicates a common trait before divergence.[99] Reptiles are therefore the most logical group to investigate the origins of sleep. Daytime activity in reptiles alternates between basking and short bouts of active behavior, which has significant neurological and physiological similarities to sleep states in mammals. It is proposed that REM sleep evolved from short bouts of motor activity in reptiles while SWS evolved from their basking state which shows similar slow wave EEG patterns.[100]
Early mammals engaged in polyphasic sleep, dividing sleep into multiple bouts per day. What then explains monophasic sleep behavior widely observed in mammals today? Higher daily sleep quotas and shorter sleep cycles in polyphasic species as compared to monophasic species, suggest that polyphasic sleep may be a less efficient means of attaining sleep’s benefits. Small species with higher BMR may therefore have less efficient sleep patterns. It follows that the evolution of monophasic sleep may hitherto be an unknown advantage of evolving larger mammalian body sizes and therefore lower BMR.[101]
Genetics
It is hypothesized that a considerable amount of sleep-related behavior, such as when and how long a person needs to sleep, is regulated by genetics. Researchers have discovered some evidence that seems to support this assumption.[102] Monozygotic (identical) but not dizygotic (fraternal) twins tend to have similar sleep habits. Neurotransmitters, molecules whose production can be traced to specific genes, are one genetic influence on sleep which can be analyzed. And the circadian clock has its own set of genes.[103] ABCC9 is one gene found which influences the duration of human sleep.[104]
Insomnia
Insomnia, a dyssomnia, is a general term describing difficulty falling asleep and staying asleep. Insomnia is the most common sleep problem, with many adults reporting occasional insomnia, and 10– 15% reporting a chronic condition.[105] Insomnia can have many different causes, including psychological stress, a poor sleep environment, an inconsistent sleep schedule, or excessive mental or physical stimulation in the hours before bedtime. Insomnia is often treated through behavioral changes like keeping a regular sleep schedule, avoiding stimulating or stressful activities before bedtime, and cutting down on stimulants such as caffeine. The sleep environment may be improved by installing heavy drapes to shut out all sunlight, and keeping computers, televisions and work materials out of the sleeping area.
A 2010 review of published scientific research suggested that exercise generally improves sleep for most people, and helps sleep disorders such as insomnia. The optimum time to exercise may be 4 to 8 hours before bedtime, though exercise at any time of day is beneficial, with the exception of heavy exercise taken shortly before bedtime, which may disturb sleep. However there is insufficient evidence to draw detailed conclusions about the relationship between exercise and sleep.[106] Sleeping medications such as Ambien and Lunesta are an increasingly popular treatment for insomnia. Although these nonbenzodiazepine medications are generally believed to be better and safer than earlier generations of sedatives, they have still generated some controversy and discussion regarding side-effects. White noise appears to be a promising treatment for insomnia.[107]
Obstructive sleep apnea
Obstructive sleep apnea is a condition in which major pauses in breathing occur during sleep, disrupting the normal progression of sleep and often causing other more severe health problems. Apneas occur when the muscles around the patient's airway relax during sleep, causing the airway to collapse and block the intake of oxygen.[108] As oxygen levels in the blood drop, the patient then comes out of deep sleep in order to resume breathing. When several of these episodes occur per hour, sleep apnea rises to a level of seriousness that may require treatment.
Diagnosing sleep apnea usually requires a professional sleep study performed in a sleep clinic, because the episodes of wakefulness caused by the disorder are extremely brief and patients usually do not remember experiencing them. Instead, many patients simply feel tired after getting several hours of sleep and have no idea why. Major risk factors for sleep apnea include chronic fatigue, old age, obesity and snoring.
Other sleep disorders
Sleep disorders include narcolepsy, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), upper airway resistance syndrome (UARS), and the circadian rhythm sleep disorders. Fatal familial insomnia, or FFI, is an extremely rare genetic disease with no known treatment or cure, is characterized by increasing insomnia as one of its symptoms; ultimately sufferers of the disease stop sleeping entirely, before dying of the disease.[58]
Somnambulism, known as sleep walking, is also a common sleeping disorder, especially among children. In somnambulism the individual gets up from his/her sleep and wanders around while still sleeping.[109]
Older people may be more easily awakened by disturbances in the environment[110] and may to some degree lose the ability to consolidate sleep.
Effect of food and drugs on sleep
Hypnotics
- Nonbenzodiazepine hypnotics such as eszopiclone (Lunesta), zaleplon (Sonata), and zolpidem (Ambien) are commonly used as sleep aids prescribed by doctors to treat forms of insomnia. Nonbenzodiazepines are the most commonly prescribed and OTC sleep aids used worldwide and have been greatly growing in use since the 1990s. They target the GABAA receptor.
- Benzodiazepines target the GABAA receptor also, and as such, they are commonly used sleep aids as well, though benzodiazepines have been found to decrease REM sleep.[111]
- Antihistamines, such as diphenhydramine (Benadryl) and doxylamine (found in various OTC medicines, such as NyQuil)
- Alcohol – Often, people start drinking alcohol in order to get to sleep (alcohol is initially a sedative and will cause somnolence, encouraging sleep).[112] However, being addicted to alcohol can lead to disrupted sleep, because alcohol has a rebound effect later in the night. As a result, there is strong evidence linking alcoholism and forms of insomnia.[113] Alcohol also reduces REM sleep.[111]
- Barbiturates cause drowsiness and have actions similar to alcohol in that they have a rebound effect and inhibit REM sleep, so they are not used as a long-term sleep aid.[114]
- Melatonin is a naturally occurring hormone that regulates sleepiness. It is made in the brain, where tryptophan is converted into serotonin and then into melatonin, which is released at night by the pineal gland to induce and maintain sleep. Melatonin supplementation may be used as a sleep aid, both as a hypnotic and as a chronobiotic (see phase response curve, PRC).
- Siesta and the "post-lunch dip" – Many people have a temporary drop in alertness in the early afternoon, commonly known as the "post-lunch dip." While a large meal can make a person feel sleepy, the post-lunch dip is mostly an effect of the biological clock. People naturally feel most sleepy (have the greatest "drive for sleep") at two times of the day about 12 hours apart—for example, at 2:00 a.m. and 2:00 p.m. At those two times, the body clock "kicks in." At about 2 p.m. (14:00), it overrides the homeostatic buildup of sleep debt, allowing several more hours of wakefulness. At about 2 a.m. (02:00), with the daily sleep debt paid off, it "kicks in" again to ensure a few more hours of sleep.
- Tryptophan – The amino acid tryptophan is a building block of proteins. It has been claimed to contribute to sleepiness, since it is a precursor of the neurotransmitter serotonin, involved in sleep regulation. However, no solid data have ever linked modest dietary changes in tryptophan to changes in sleep.
- Cannabis – Some people use cannabis to induce sleepiness. Users often report relaxation and drowsiness. It has been shown that tetrahydrocannabinol (THC), the principal psychoactive constituent in cannabis, reduces the amount of REM sleep.[115] Frequent users often report being unable to recall their dreams.
Stimulants
- Amphetamine (dextroamphetamine, and a related, slightly more powerful drug methamphetamine, etc.) are used to treat narcolepsy. Their most common effects are anxiety, insomnia, stimulation, increased alertness, and decreased hunger.
- Caffeine is a stimulant that works by slowing the action of the hormones in the brain that cause somnolence, particularly by acting as an antagonist at adenosine receptors. Effective dosage is individual, in part dependent on prior usage. It can cause a rapid reduction in alertness as it wears off.
- Cocaine and crack cocaine – Studies on cocaine have shown its effects to be mediated through the circadian rhythm system.[116] This may be related to the onset of hypersomnia (oversleeping) in regard to "cocaine-induced sleep disorder."[117]
- MDMA, including similar drugs like MDA, MMDA, or bk-MDMA – The class of drugs called empathogen-entactogens keep users awake with intense euphoria.
- Methylphenidate – Commonly known by the brand names Ritalin and Concerta, methylphenidate is similar in action to amphetamine and cocaine; its chemical composition more closely resembles that of cocaine.
- Tobacco – Tobacco has been found not only to disrupt but also to reduce total sleep time. In studies, users have described more daytime drowsiness than nonsmokers.[118]
- Other analeptic drugs like Modafinil and Armodafinil are prescribed to treat narcolepsy, idiopathic hypersomnia, shift work sleep disorder, and other conditions causing Excessive Daytime Sleepiness. The precise mechanism of these CNS stimulants is not known, but they have been shown to increase both the release of monoamines and levels of hypothalamic histamine, thereby promoting wakefulness.
Nutritional effects on sleep
Dietary and nutritional choices affect sleep duration and quality. Research is being conducted in an attempt to discover what kinds of nutritional choices result in better sleep quality.
A study in the Western Journal of Nursing Research in 2011[119] compared how sleep quality was affected by four different diets: a high protein diet, a high fat diet, a high carbohydrate diet, and a control diet. Results indicated that the diets high in protein resulted in fewer wakeful episodes during night-time sleep. The high carbohydrate diet was linked to much shorter periods of quiescent or restful sleep. These results suggest that ingested nutrients do play a role in determining sleep quality. Another investigation published in Nutrition Research in 2012[120] examined the effects of various combinations of dietary choices in regard to sleep. Although it is difficult to determine one perfect diet for sleep enhancement, this study indicated that a variety of micro and macro nutrients are needed to maintain levels of healthful and restful sleep. A varied diet containing fresh fruits and vegetables, low-fat proteins, and whole grains can be the best nutritional option for individuals seeking to improve the quality of their sleep.
Anthropology of sleep

Research suggests that sleep patterns vary significantly across cultures.[121][122] The most striking differences are between societies that have plentiful sources of artificial light and ones that do not.[121] The primary difference appears to be that pre-light cultures have more broken-up sleep patterns.[121] For example, people without artificial light might go to sleep far sooner after the sun sets, but then wake up several times throughout the night, punctuating their sleep with periods of wakefulness, perhaps lasting several hours.[121]
The boundaries between sleeping and waking are blurred in these societies.[121] Some observers believe that nighttime sleep in these societies is most often split into two main periods, the first characterized primarily by deep sleep and the second by REM sleep.[121]
Some societies display a fragmented sleep pattern in which people sleep at all times of the day and night for shorter periods. In many nomadic or hunter-gatherer societies, people will sleep on and off throughout the day or night depending on what is happening.[121] Plentiful artificial light has been available in the industrialized West since at least the mid-19th century, and sleep patterns have changed significantly everywhere that lighting has been introduced.[121] In general, people sleep in a more concentrated burst through the night, going to sleep much later, although this is not always true.[121]
Historian Roger Ekrich thinks that the traditional pattern of "segmented sleep," as it is called, began to disappear among the urban upper class in Europe in the late 17th century and the change spread over the next 200 years; by the 1920s "the idea of a first and second sleep had receded entirely from our social consciousness."[123] Ekrich attributes the change to increases in "street lighting, domestic lighting and a surge in coffee houses," which slowly made nighttime a legitimate time for activity, decreasing the time available for rest.[123] Today in most societies people sleep during the night, but in very hot climates they may sleep during the day.[124] During Ramadan, many Muslims sleep during the day rather than at night[125] and people working nights try to sleep in the daytime.
In some societies, people generally sleep with at least one other person (sometimes many) or with animals. In other cultures, people rarely sleep with anyone but a most intimate relation, such as a spouse. In almost all societies, sleeping partners are strongly regulated by social standards. For example, a person might only sleep with the immediate family, the extended family, a spouse or romantic partner, children, children of a certain age, children of specific gender, peers of a certain gender, friends, peers of equal social rank, or with no one at all. Sleep may be an actively social time, depending on the sleep groupings, with no constraints on noise or activity.[121]
People sleep in a variety of locations. Some sleep directly on the ground; others on a skin or blanket; others sleep on platforms or beds. Some sleep with blankets, some with pillows, some with simple headrests, some with no head support. These choices are shaped by a variety of factors, such as climate, protection from predators, housing type, technology, personal preference, and the incidence of pests.[121]
Sleep in other animals

Neurological sleep states can be difficult to detect in some animals. In these cases, sleep may be defined using behavioral characteristics such as minimal movement, postures typical for the species, and reduced responsiveness to external stimulation. Sleep is quickly reversible, as opposed to hibernation or coma, and sleep deprivation is followed by longer or deeper rebound sleep. Herbivores, who require a long waking period to gather and consume their diet, typically sleep less each day than similarly sized carnivores, who might well consume several days' supply of meat in a sitting.
Horses and other herbivorous ungulates can sleep while standing, but must necessarily lie down for REM sleep (which causes muscular atony) for short periods. Giraffes, for example, only need to lie down for REM sleep for a few minutes at a time. Bats sleep while hanging upside down. Some aquatic mammals and some birds can sleep with one half of the brain while the other half is awake, so-called unihemispheric slow-wave sleep.[126] Birds and mammals have cycles of non-REM and REM sleep (as described above for humans), though birds' cycles are much shorter and they do not lose muscle tone (go limp) to the extent that most mammals do.
Many mammals sleep for a large proportion of each 24-hour period when they are very young.[127] However, killer whales and some other dolphins do not sleep during the first month of life.[128] Instead, young dolphins and whales frequently take rests by pressing their body next to their mother’s while she swims. As the mother swims she is keeping her offspring afloat to prevent them from drowning. This allows young dolphins and whales to rest, which will help keep their immune system healthy; in turn, protecting them from illnesses.[129] During this period, mothers often sacrifice sleep for the protection of their young from predators. However, unlike other mammals, adult dolphins and whales are able to go without sleep for a month.[129][130]
Also unlike terrestrial mammals, dolphins, whales, and pinnipeds (seals) cannot go into a deep sleep. The consequences of falling into a deep sleep for marine mammalian species can be suffocation and drowning, or becoming easy prey for predators. Thus, dolphins, whales, and seals engage in unihemispheric sleep, which allows one brain hemisphere to remain fully functional, while the other goes to sleep. The hemisphere that is asleep, alternates so that both hemispheres can be fully rested.[129][131]
Just like terrestrial mammals, pinnipeds that sleep on land fall into a deep sleep and both hemispheres of their brain shut down and are in full sleep mode.[132][133]
- Sleeping in art
-
Zwei schlafende Mädchen auf der Ofenbank, Albert Anker, 1895
-
Lullaby, William-Adolphe Bouguereau, 1875
-
Flaming June by Frederic Lord Leighton, ~1895
-
The Sentry by Carel Fabritius, 1654
-
Sleeping Jaguar, de
-
Shrapek (Snorer), Wrocław's dwarfs
-
The sleep of reason produces monsters by Goya, 1799
See also
Positions, practices, and rituals
References
- ^ Macmillan Dictionary for Students Macmillan, Pan Ltd. (1981), p. 936. Retrieved 1 October 2009.
- ^ Bingham, Roger (February 2007). "Waking Up To Sleep" (Several conference videos). The Science Network. Retrieved 25 January 2008.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b "Brain Basics: Understanding Sleep". nih.gov.
- ^ J. Alan Hobson, Edward F. Pace-Scott, & Robert Stickgold (2000), “Dreaming and the brain: Toward a cognitive neuroscience of conscious states”, Behavioral and Brain Sciences 23.
- ^ J. Alan Hobson & Robert W. McCarley, “The Brain as a Dream-State Generator: An Activation-Synthesis Hypothesis of the Dream Process”, American Journal of Psychiatry 134.12, December 1977.
- ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, p. 1118–1119. “Compared with wakefulness, sleep reduces brain energy demands, as suggested by the 44% reduction in the cerebral metabolic rate (CMR) of glucose (791) and a 25% reduction in the CMR of O2 (774) during sleep.”
- ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, p. 1087.
- ^ Parmeggiani (2011), Systemic Homeostasis and Poikilostasis in Sleep, p. 9–11.
- ^ Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C (March 2007). "The visual scoring of sleep in adults" (PDF). Journal of Clinical Sleep Medicine. 3 (2): 121–31. PMID 17557422.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Brown et al. (2012), “Control of Sleep and Wakefulness”, pp. 1108–1109.
- ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, pp. 1100–1102.
- ^ Parmeggiani (2011), Systemic Homeostasis and Poikilostasis in Sleep, passim.
- ^ a b Schacter, Daniel L.; Gilbert, Daniel T. and Wegner, Daniel M. (2009) Psychology, Worth Publishers, ISBN 1429206152
- ^ a b Saladin, Kenneth S. (2012). Anatomy and Physiology: The Unity of Form and Function, 6th Edition. McGraw-Hill. p. 537. ISBN 978-0-07-337825-1.
- ^ Carlson NR, Miller HL, Heth DS, Donahoe JW, Martin GN (2010). Psychology The Science of Behavior, Books a La Carte Edition. Pearson College Div. ISBN 0205762239.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ David G. Myers (22 September 2003). Psychology, Seventh Edition, in Modules (High School Version). Macmillan. pp. 268–. ISBN 978-0-7167-8595-8. Retrieved 22 August 2012.
- ^ Siegel, Jerome M (1999). "Sleep". Encarta Encyclopedia. Microsoft. Archived from the original on 14 December 2007. Retrieved 25 January 2008.
- ^ Fenton, Reuven (29 August 2007). "Bio-alarm clocks set for perfect wake-up". Reuters. Retrieved 9 June 2008.
- ^ Loomis AL, Harvey EN, Hobart GA (1937). "III Cerebral states during sleep, as studied by human brain potentials". J Exp Psychol. 21 (2): 127–44. doi:10.1037/h0057431.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dement W, Kleitman N (1957). "Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming". Electroencephalogr Clin Neurophysiol. 9 (4): 673–90. doi:10.1016/0013-4694(57)90088-3. PMID 13480240.
- ^ Rechtschaffen A, Kales A, editors. (1968). A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (PDF). Washington: Public Health Service, US Government Printing Office.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Iber, C; Ancoli-Israel, S; Chesson, A; Quan, SF for the American Academy of Sleep Medicine (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester: American Academy of Sleep Medicine.
- ^ Psychology World (1998). "Stages of Sleep" (PDF). Retrieved 15 June 2008.
(includes illustrations of "sleep spindles" and "K-complexes")
- ^ Schulz H (April 2008). "Rethinking sleep analysis". Journal of Clinical Sleep Medicine. 4 (2): 99–103. PMC 2335403. PMID 18468306.
- ^ Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ (1 October 1999). "Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day". Am J Physiol. 277 (4): R1152–R1163. PMID 10516257.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D (2007). "Siesta in healthy adults and coronary mortality in the general population". Archives of Internal Medicine. 167 (3): 296–301. doi:10.1001/archinte.167.3.296. PMID 17296887.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Zaregarizi M, Edwards B, George K, Harrison Y, Jones H, Atkinson G (2007). "Acute changes in cardiovascular function during the onset period of daytime sleep: comparison to lying awake and standing". Journal of Applied Physiology (Bethesda, Md. : 1985). 103 (4): 1332–8. doi:10.1152/japplphysiol.00474.2007. PMID 17641220.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b MohammadReza Zaregarizi. Effects of Exercise & Daytime Sleep on Human Haemodynamics: With Focus on Changes in Cardiovascular Function during Daytime Sleep Onset. ISBN 978-3-8484-1726-1.
- ^ Kaul P, Passafiume J, Sargent CR, O'Hara BF (2010). "Meditation acutely improves psychomotor vigilance, and may decrease sleep need". Behav Brain Funct. 6: 47. doi:10.1186/1744-9081-6-47. PMC 2919439. PMID 20670413.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, pp. 1134–1138.
- ^ Zisapel N (2007). "Sleep and sleep disturbances: biological basis and clinical implications". Cell Mol Life Sci. 64 (10): 1174–86. doi:10.1007/s00018-007-6529-9. PMID 17364142.
- ^ a b de Benedictis, Tina, PhD (2007). "Understanding Sleep: Sleep Needs, Cycles, and Stages". Helpguide.org. Retrieved 25 January 2008.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ "Gene Cuts Need for Sleep - Sleep Disorders Including, Sleep Apnea, Narcolepsy, Insomnia, Snoring and Nightmares on MedicineNet.com". Archived from the original on 14 July 2011. Retrieved 11 June 2010.
- ^ He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Rossner MJ, Nishino S, Fu YH (2009). "The transcriptional repressor DEC2 regulates sleep length in mammals". Science. 325 (5942): 866–70. doi:10.1126/science.1174443. PMC 2884988. PMID 19679812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gottselig JM, Adam M, Rétey JV, Khatami R, Achermann P, Landolt HP (March 2006). "Random number generation during sleep deprivation: effects of caffeine on response maintenance and stereotypy". Journal of Sleep Research. 15 (1): 31–40. doi:10.1111/j.1365-2869.2006.00497.x. PMID 16490000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Iglowstein I, Jenni OG, Molinari L, Largo RH (February 2003). "Sleep duration from infancy to adolescence: reference values and generational trends". Pediatrics. 111 (2): 302–7. doi:10.1542/peds.111.2.302. PMID 12563055.
Thus, the shift in the evening bedtime across cohorts accounted for the substantial decrease in sleep duration in younger children between the 1970s and the 1990s... [A] more liberal parental attitude toward evening bedtime in the past decades is most likely responsible for the bedtime shift and for the decline of sleep duration...
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Molecules that build up and make you sleep. thebrain.mcgill.ca
- ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, pp. 1113–1114. “Adenosine levels correlate with time spent awake. Endogenous, extracellular adenosine levels in the BF (102, 591, 843, 1017, 1018) and cortex (591, 1017) increase in proportion with time spent awake. Thus adenosine induces sleep and adenosine levels track sleep need, fulfilling the criteria for adenosine being a homeostatic sleep factor.”
- ^ Reference list is found on image page in Commons: Commons:File:Effects of sleep deprivation.svg#References
- ^ Dijk DJ, Lockley SW (February 2002). "Functional Genomics of Sleep and Circadian Rhythm Invited Review: Integration of human sleep-wake regulation and circadian rhythmicity". J Appl Physiol. 92 (2): 852–62. doi:10.1152/japplphysiol.00924.2001. PMID 11796701.
Consolidation of sleep for 8 h or more is only observed when sleep is initiated ~6–8 h before the temperature nadir.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ (1 October 1999). "Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day". Am J Physiol. 277 (4): R1152–R1163. PMID 10516257.
... significant homeostatic and circadian modulation of sleep structure, with the highest sleep efficiency occurring in sleep episodes bracketing the melatonin maximum and core body temperature minimum
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ (2008). "Self-Reported and Measured Sleep Duration: How Similar Are They?". Epidemiology. 19 (6): 838–45. doi:10.1097/EDE.0b013e318187a7b0. PMC 2785092. PMID 18854708.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Insomnia Causes. Healthcommunities.com. Original Publication: 1 December 2000, Updated: 1 December 2007.
- ^ Rhonda Rowland (15 February 2002). "Experts challenge study linking sleep, life span". CNN. Retrieved 29 October 2013.
- ^ Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB (May 2004). "A prospective study of sleep duration and mortality risk in women". Sleep. 27 (3): 440–4. PMID 15164896.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB (July 2006). "Correlates of long sleep duration". Sleep. 29 (7): 881–9. PMC 3500381. PMID 16895254.
{{cite journal}}: CS1 maint: multiple names: authors list (link); cf. Irwin MR, Ziegler M (February 2005). "Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics". Hypertension. 45 (2): 252–7. doi:10.1161/01.HYP.0000153517.44295.07. PMID 15642774. - ^ Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG (December 2007). "A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort". Sleep. 30 (12): 1659–66. PMC 2276139. PMID 18246975.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thase ME (2006). "Depression and sleep: pathophysiology and treatment" (Free full text). Dialogues in clinical neuroscience. 8 (2): 217–226. ISSN 1294-8322. PMC 3181772. PMID 16889107.
- ^ Mann, Joseph John; David J. Kupfer (1993). Biology of Depressive Disorders: Subtypes of depression and comorbid disorders, Part 2 (Google books). Springer. p. 49. ISBN 0-306-44296-5. Retrieved 24 July 2009.
- ^ Dahl RE (2009). "The regulation of sleep and arousal: Development and psychopathology". Development and Psychopathology. 8 (01): 3–27. doi:10.1017/S0954579400006945.
- ^ Jenni OG, Dahl RE (2008). "Sleep, cognition, and neuron, and emotion: A developmental review.". In Nelson CA, Luciana M (ed.). Handbook of developmental cognitive neuroscience (2nd ed.). Cambridge, Mass.: MIT Press. pp. 807–817. ISBN 0262141043.
- ^ a b Scher A (2005). "Infant sleep at 10 months of age as a window to cognitive development". Early Human Development. 81 (3): 289–92. doi:10.1016/j.earlhumdev.2004.07.005. PMID 15814211.
- ^ Spruyt K, Aitken RJ, So K, Charlton M, Adamson TM, Horne RS (2008). "Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life". Early Human Development. 84 (5): 289–96. doi:10.1016/j.earlhumdev.2007.07.002. PMID 17707119.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Bernier A, Carlson SM, Bordeleau S, Carrier J (2010). "Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning". Child Development. 81 (6): 1739–52. doi:10.1111/j.1467-8624.2010.01507.x. PMID 21077861.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hupbach A, Gomez RL, Bootzin RR, Nadel L (2009). "Nap-dependent learning in infants". Developmental Science. 12 (6): 1007–12. doi:10.1111/j.1467-7687.2009.00837.x. PMID 19840054.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h Hirshkowitz, Max ; Whiton, Kaitlyn; et al. (14 January 2015). "National Sleep Foundation's sleep time duration recommendations: methodology and results summary". Sleep Health: Journal of the National Sleep Foundation. Elsevier Inc. doi:10.1016/j.sleh.2014.12.010. Retrieved 4 February 2015.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Backgrounder: Later School Start Times". National Sleep Foundation. n.d. Retrieved 2 October 2009.
Teens are among those least likely to get enough sleep; while they need on average 91⁄4 hours of sleep per night...
- ^ a b Max, D. T. The Secrets of Sleep National Geographic Magazine, May 2010.
- ^ Cirelli C, Tononi G (26 August 2008). "Is Sleep Essential?". PLoS Biol. 6 (8). Public Library of Science: e216. doi:10.1371/journal.pbio.0060216. PMC 2525690. PMID 18752355.
... it would seem that searching for a core function of sleep, particularly at the cellular level, remains a worthwhile exercise
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Sleep Syllabus. B. The Phylogeny of Sleep". Sleep Research Society, Education Committee. Retrieved 26 September 2010.
- ^ "Function of Sleep.". Scribd.com. Retrieved on 1 December 2011.
- ^ a b Daan S, Barnes BM, Strijkstra AM (1991). "Warming up for sleep? Ground squirrels sleep during arousals from hibernation". Neurosci. Lett. 128 (2): 265–8. doi:10.1016/0304-3940(91)90276-Y. PMID 1945046.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid1945046" was defined multiple times with different content (see the help page). - ^ "Sleep Drives Metabolite Clearance from the Adult Brain". Science. 342 (6156): 373–377. 2013. doi:10.1126/science.1241224. PMID 24136970. Retrieved 18 October 2013.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Gümüştekín K, Seven B, Karabulut N, Aktaş O, Gürsan N, Aslan S, Keleş M, Varoglu E, Dane S (2004). "Effects of sleep deprivation, nicotine, and selenium on wound healing in rats". Int J Neurosci. 114 (11): 1433–42. doi:10.1080/00207450490509168. PMID 15636354.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S (2007). "Effects of acute and chronic sleep loss on immune modulation of rats". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 293 (1): R504–9. doi:10.1152/ajpregu.00105.2007. PMID 17409265.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Opp MR (January 2009). "Sleeping to fuel the immune system: mammalian sleep and resistance to parasites" (Full text, Creative Commons Attribution License). BMC Evolutionary Biology. 9. BioMed Central Ltd.: 1471–2148. doi:10.1186/1471-2148-9-8. PMC 2633283. PMID 19134176.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Peres, Judy (14 March 2012) A good reason to get your zzz's Chicago Tribune Health, retrieved 26 March 2014
- ^ Reimund E (October 1994). "The free radical flux theory of sleep". Medical Hypotheses. 45 (4): 231–3. doi:10.1016/0306-9877(94)90071-X. PMID 7838006.
- ^ Jenni OG, Molinari L, Caflisch JA, Largo RH (2007). "Sleep duration from ages 1 to 10 years: Variability and stability in comparison with growth". Pediatrics. 120 (4): e769–e776. doi:10.1542/peds.2006-3300. PMID 17908734.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Van Cauter E, Leproult R, Plat L (2000). "Age-related changes in slow-wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men". Journal of the American Medical Association. 284 (7): 861–868. doi:10.1001/jama.284.7.861. PMID 10938176.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Brain may flush out toxins during sleep". National Institutes of Health. Retrieved 25 October 2013.
- ^ Siegel JM (2005). "Clues to the functions of mammalian sleep". Nature. 437 (7063): 1264–1271. Bibcode:2005Natur.437.1264S. doi:10.1038/nature04285. PMID 16251951.
- ^ Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Institute for Laboratory Animal Research (ILAR), National Research Council. The National Academies Press. 2003. p. 121. ISBN 978-0-309-08903-6.
Sleep deprivation of over 7 days with the disk-over-water system results in the development of ulcerative skin lesions, hyperphagia, loss of body mass, hypothermia, and eventually septicemia and death in rats (Everson, 1995; Rechtschaffen et al., 1983).
{{cite book}}: CS1 maint: others (link) - ^ Guilio Tononi and Chiara Cirelli. "Perchance to Prune" Scientific American. August 2013. Pgs 34-39. Print.
- ^ Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP (1995). "A functional role for REM sleep in brain maturation". Behavioural Brain Research. 69 (1–2): 1–11. doi:10.1016/0166-4328(95)00018-o. PMID 7546299.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mirmiran M, Scholtens J, van de Poll NE, Uylings HB, van der Gugten J, Boer GJ (April 1983). "Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat". Brain Research. 283 (2–3): 277–86. doi:10.1016/0165-3806(83)90184-0. PMID 6850353.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morrissey MJ, Duntley SP, Anch AM, Nonneman R (2004). "Active sleep and its role in the prevention of apoptosis in the developing brain". Medical Hypotheses. 62 (6): 876–9. doi:10.1016/j.mehy.2004.01.014. PMID 15142640.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Amanda Schaffer (27 May 2007). "Why do we Sleep?". Slate.com. Retrieved 23 August 2008.
- ^ Turner TH, Drummond SP, Salamat JS, Brown GG (2007). "Effects of 42 hr sleep deprivation on component processes of verbal working memory". Neuropsychology. 21 (6): 787–795. doi:10.1037/0894-4105.21.6.787. PMID 17983292.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Daltrozzo J, Claude L, Tillmann B, Bastuji H, Perrin F (2012). Zang, Yu-Feng (ed.). "Working Memory Is Partially Preserved during Sleep". PLoS ONE. 7 (12): e50997. doi:10.1371/journal.pone.0050997. PMC 3517624. PMID 23236418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ cited in Born J, Rasch B, Gais S (2006). "Sleep to remember". Neuroscientist. 12 (5): 410–24. doi:10.1177/1073858406292647. PMID 16957003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Datta S (2000). "Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity". The Journal of Neuroscience. 20 (22): 8607–8613. PMID 11069969.
- ^ Kudrimoti HS, Barnes CA, McNaughton BL (1999). "Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics". The Journal of Neuroscience. 19 (10): 4090–4101. PMID 10234037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marshall et al., 2006, as cited in Walker MP (2009). "The role of sleep in cognition and emotion". Annals of the New York Academy of Sciences. 1156: 168–97. doi:10.1111/j.1749-6632.2009.04416.x. PMID 19338508.
- ^ Saper CB, Scammell TE, Lu J (2005). "Hypothalamic regulation of sleep and circadian rhythms". Nature. 437 (7063): 1257–63. doi:10.1038/nature04284. PMID 16251950.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stickgold R (2005). "Sleep-dependent memory consolidation". Nature. 437 (7063): 1272–8. doi:10.1038/nature04286. PMID 16251952.
- ^ Capellini I, McNamara P, Preston BT, Nunn CL, Barton RA (2009). Sporns, Olaf (ed.). "Does sleep play a role in memory consolidation? A comparative test". PLoS ONE. 4 (2): 4609. Bibcode:2009PLoSO...4.4609C. doi:10.1371/journal.pone.0004609. PMC 2643482. PMID 19240803.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Cirelli C, Gutierrez CM, Tononi G (2004). "Extensive and divergent effects of sleep and wakefulness on brain gene expression". Neuron. 41 (1): 35–43. doi:10.1016/S0896-6273(03)00814-6. PMID 14715133.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Choi, Charles Q. (25 August 2009) New Theory Questions Why We Sleep, LiveScience.com.
- ^ "BPS Research Digest: An afternoon nap tunes out negative emotions, tunes in positive ones". bps.org.uk.
- ^ Hock, R. R. (2013). To sleep, no doubt to dream… In Forty studies that changed psychology (7th ed., pp. 42–49). Upper Saddle River, NJ: Pearson Education. ISBN 0205918395.
- ^ William Dement, “The Effect of Dream Deprivation: The need for a certain amount of dreaming each night is suggested by recent experiments.” Science 131.3415, 10 June 1960.
- ^ Naiman, Rubin (2007). "How To Interpret Your Dreams". Allure. 17 (5): n/a.
- ^ Pinel, John P. J. (2011). Biopsychology, 8th Edition. Pearson Education, Inc. p. 359. ISBN 978-0-205-83256-9.
- ^ Connor, Steve (3 April 2009). "Revealed: why we need a good night's sleep". The Independent. Retrieved 2 December 2010.
- ^ Hobson JA, McCarley RW (1977). "The brain as a dream state generator: An activation-synthesis hypothesis of the dream process". American Journal of Psychiatry. 134 (12): 1335–1348. PMID 21570.
- ^ Tsoukalas, Ioannis (2012). "The origin of REM sleep: A hypothesis". Dreaming. 22 (4): 253. doi:10.1037/a0030790.
- ^ Vitelli, R. (2013). Exploring the Mystery of REM Sleep. Psychology Today, On-line blog, 25 March
- ^ Low PS, Shank SS, Sejnowski TJ, Margoliash D (2008). "Mammalian-like features of sleep structure in zebra finches". Proceedings of the National Academy of Sciences of the United States of America. 105 (26): 9081–9086. Bibcode:2008PNAS..105.9081L. doi:10.1073/pnas.0703452105. PMC 2440357. PMID 18579776.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rial RV, Akaârir M, Gamundí A, Nicolau C, Garau C, Aparicio S, Tejada S, Gené L, González J, De Vera LM, Coenen AM, Barceló P, Esteban S (2010). "Evolution of wakefulness, sleep and hibernation: From reptiles to mammals". Neuroscience and Biobehavioral Reviews. 34 (8): 1144–1160. doi:10.1016/j.neubiorev.2010.01.008. PMID 20109487.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Capellini I, Nunn CL, McNamara P, Preston BT, Barton RA (2008). "Energetic constraints, not predation, influence the evolution of sleep patterning in mammals". Functional Ecology. 22 (5): 847–853. doi:10.1111/j.1365-2435.2008.01449.x. PMC 2860325. PMID 20428321.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Rossner MJ, Nishino S, Fu YH (2009). "The Transcriptional Repressor DEC2 Regulates Sleep Length in Mammals". Science. 325 (5942): 866–70. doi:10.1126/science.1174443. PMC 2884988. PMID 19679812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, pp. 1138–1102.
- ^ "The ABCC9 of Sleep: A Genetic Factor Regulates How Long We Sleep". Science Daily. Retrieved 21 August 2012.
- ^ Brown et al. (2012), “Control of Sleep and Wakefulness”, pp. 1146–1147.
- ^ Buman, M.P. and King, A.C.: "Exercise as a Treatment to Enhance Sleep", American Journal of Lifestyle Medicine, Nov–Dec 2010.
- ^ López HH, Bracha AS, Bracha HS (2002). "Evidence based complementary intervention for insomnia" (PDF). Hawaii Med J. 61 (9): 192, 213. PMID 12422383.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "What is Sleep Apnoea? (Sleep Apnea)". britishsnoring.co.uk.
- ^ Dugdale, David, C. (22 May 2011). Sleepwalking. US National institutes of health.
- ^ How Aging Changes Sleep Patterns by Allison Aubrey. Morning Edition, 3 Aug 2009.
- ^ a b Lee-chiong, Teofilo (24 April 2008). Sleep Medicine: Essentials and Review. Oxford University Press, USA. p. 52. ISBN 0-19-530659-7.
- ^ Alcohol and Sleep. Sleepdex.org. Retrieved on 1 December 2011.
- ^ Alcohol and Sleep. Alcoholism.about.com (10 January 2011). Retrieved on 1 December 2011.
- ^ Sleep Medications: Barbituates. Sleepdex.org. Retrieved on 1 December 2011.
- ^ Marijuana, Sleep and Dreams. psychologytoday.com. Retrieved on 10 February 2012.
- ^ Abarca C, Albrecht U, Spanagel R (June 2002). "Cocaine sensitization and reward are under the influence of circadian genes and rhythm". Proceedings of the National Academy of Sciences of the United States of America. 99 (13): 9026–30. doi:10.1073/pnas.142039099. PMC 124417. PMID 12084940.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Primary hypersomnia: Diagnostic Features. mindsite.com
- ^ Causes of Sleep Deprivation. Sleep.com.
- ^ Lindseth, Gelinda, Paul Lindseth, and Mark Thompson. "Nutritional Effects on Sleep."Western Journal of Nursing Research (2011): n. pag. Web.
- ^ Peuhkuri, Katri, Nora Sihvola, and Riitta Korpela. "Diet Promotes Sleep Duration and Quality." Nutrition Research 32.5 (2012): 309-19. Print.
- ^ a b c d e f g h i j k Carol M. Worthman and Melissa K. Melby. "6. Toward a comparative developmental ecology of human sleep". A comparative developmental ecology. Emory University.
{{cite book}}:|format=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Slumber's Unexplored Landscape. Science News Online (25 September 1999). Retrieved on 1 December 2011.
- ^ a b Hegarty, Stephanie (22 February 2012). "The myth of the eight-hour sleep". BBC News. Retrieved 22 February 2012.
- ^ Ellsworth Huntington, Civilization and Climate - Page 126
- ^ Dilara Hafiz, Imran Hafiz, Yasmine Hafiz (2009). The American Muslim Teenager's Handbook. ISBN 978-1416986997.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Mukhametov LM, Supin AY, Polyakova IG (14 October 1977). "Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins". Brain Research. 134 (3): 581–584. doi:10.1016/0006-8993(77)90835-6. PMID 902119.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Faraco, Juliette (1 August 2000). "Re: Are there animals who don't sleep or that sleep very little?". MadSci Network: Zoology. Retrieved 25 January 2008.
- ^ The giraffe only sleeps 2 hours a day in about 5–15 minute sessions. Koalas are the longest sleeping-mammals, about 20–22 hours a day.Insomnia Mania: Newborn Mammals Don't Sleep for a Month. LiveScience.com
- ^ a b c Hecker, Bruce (2 February 1998). "How do Whales and Dolphins Sleep without Drowning?". Scientific American. mirror
- ^ Britt, Robert (29 June 2005). "Insomnia Mania: Newborn Mammals Don't Sleep for a Month". Live Science.
- ^ "Seals Sleep with Only Half of Their Brain at a Time". Oceana.org. 12 March 2013.
- ^ Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM, Siegel JM (2007). "Cortical Acetylcholine Release Is Lateralized during Asymmetrical Slow-Wave Sleep in Northern Fur Seals". The Journal of Neuroscience. 27 (44): 11999–12006. doi:10.1523/JNEUROSCI.2968-07.2007. PMID 17978041.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Study Seals Sleep with Half Their Brain". upi.com. 19 February 2013.
Sources
- Brown, Ritchie E., Radhika Basheer, James T. McKenna, Robert E. Strecker, & Robert W. McCarley (2012). “Control of Sleep and Wakefulness”. Physiological Review 92, pp. 1087–1187.
- Parmeggiani, Pier Luigi (2011). Systemic Homeostasis and Poikilostasis in Sleep: Is REM Sleep a Physiological Paradox? London: Imperial College Press. ISBN 978-1-94916-572-2
External links
- nih.gov, National Center on Sleep Disorders Research
- National Sleep Foundation, information on School Start Time and Sleep at National Sleep Foundation
- HealthySleep.med.harvard.edu, from the Division of Sleep Medicine at Harvard Medical School and WGBH Educational Foundation
- PLOSjournals.org, Is Sleep Essential? by Chiara Cirelli and Giulio Tononi, from the Public Library of Science, Biology
- New York Times, 2012; "Rethinking Sleep"
- Sleep.com, an informational database offering sleep-related articles and resources.





![Sleeping Jaguar, de [Paul Klimsch]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/67/Paul_Klimsch_Schlafender_Jaguar.jpg/154px-Paul_Klimsch_Schlafender_Jaguar.jpg)